Themed collection A Decade of Progress in Click Reactions Based on CuAAC

1,2,3-Triazoles derived from olanzapine: their synthesis via an ultrasound assisted CuAAC method and evaluation as inhibitors of PDE4B
An ultrasound assisted CuAAC method afforded novel 1,2,3-triazoles derived from olanzapine as inhibitors of PDE4B.
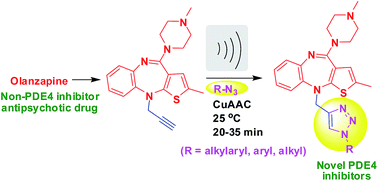
RSC Adv., 2015,5, 94623-94628
https://doi.org/10.1039/C5RA20380E
Zebrafish based strategy for the identification of a potential pharmacophore for apoptosis: a greener CuAAC approach for novel 1,2,3-triazoles derived from mefenamic acid
A novel pharmacophore for apoptosis has been identified via a screening strategy in zebrafish.

RSC Adv., 2014,4, 4878-4882
https://doi.org/10.1039/C3RA46185H
[3+2] click chemistry approach to tetrazine containing polymers
We report a [3+2] cycloaddition using 3,6-bis-propargyloxy-1,2,4,5-tetrazine and azides to synthesize energetic polymers containing 1,2,4,5-tetrazine within the scaffold.
![Graphical abstract: [3+2] click chemistry approach to tetrazine containing polymers](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2RA05339J&imageInfo.ImageIdentifier.Year=2022)
RSC Adv., 2022,12, 28490-28493
https://doi.org/10.1039/D2RA05339J
Structure-based design of 5′-substituted 1,2,3-triazolylated oseltamivir derivatives as potent influenza neuraminidase inhibitors
Exploring influenza neuraminidase inhibitors by targeting the charged residues near the entrance of the 150-cavity.

RSC Adv., 2021,11, 9528-9541
https://doi.org/10.1039/D1RA00472G
A click-based modular approach to introduction of peroxides onto molecules and nanostructures
Copper-promoted azide/alkyne cycloadditions (CuAAC) are explored as a tool for modular introduction of peroxides onto molecules and nanomaterials.

RSC Adv., 2020,10, 44408-44429
https://doi.org/10.1039/D0RA09088C
Cu(II)-alginate-based superporous hydrogel catalyst for click chemistry azide–alkyne cycloaddition type reactions in water
The Cu(II)-alginate-based superporous hydrogel was prepared and used as a heterogenous catalyst in the regioselective click of 1,4-disubstituted-1,2,3-triazoles by CuAAC reactions.

RSC Adv., 2020,10, 32821-32832
https://doi.org/10.1039/D0RA06410F
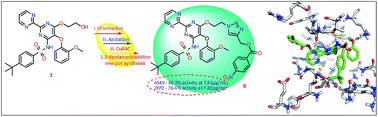
Design, synthesis, biological evaluation and molecular docking study of novel pyridoxine–triazoles as anti-Alzheimer's agents
A series of multi-target natural product-pyridoxine based derivatives were designed, synthesized, characterized and evaluated as anti-Alzheimer agents. Out of all the molecules of the series, 5i was found to be best.
RSC Adv., 2020,10, 26006-26021
https://doi.org/10.1039/D0RA04942E
Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1,2,3-triazoles
Coumarin-based 1,4-disubstituted 1,2,3-triazole derivatives were synthesized using a highly efficient, eco-friendly protocol via a copper(I)-catalyzed click reaction between various substituted arylazides and terminal alkynes.
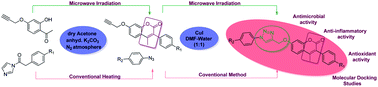
RSC Adv., 2020,10, 11615-11623
https://doi.org/10.1039/D0RA01052A
Quinoline-triazole hybrids inhibit falcipain-2 and arrest the development of Plasmodium falciparum at the trophozoite stage
The present study involves development of novel quinoline triazole-containing cysteine protease inhibitors which arrest the development of P. falciparum at the trophozoite stage.
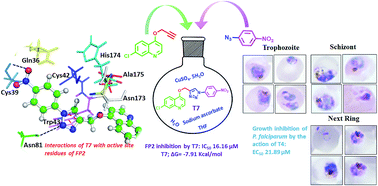
RSC Adv., 2019,9, 39410-39421
https://doi.org/10.1039/C9RA06571G
Clickable poly-L-lysine for the formation of biorecognition surfaces
The fast and stable adsorption of modified PLL on activated surfaces was combined with the versatile catalyst-free click chemistry for the fast and selective functionalization of substrates with DNA.

RSC Adv., 2019,9, 35608-35613
https://doi.org/10.1039/C9RA08714A
Discovery of novel 1,4-disubstituted 1,2,3-triazole phenylalanine derivatives as HIV-1 capsid inhibitors
Novel phenylalanine derivatives were discovered as HIV-1 capsid protein inhibitors via “click reaction”. Most of them exhibited remarkable anti-HIV-1 activity.
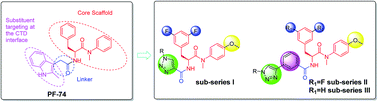
RSC Adv., 2019,9, 28961-28986
https://doi.org/10.1039/C9RA05869A
New N-phenylacetamide-incorporated 1,2,3-triazoles: [Et3NH][OAc]-mediated efficient synthesis and biological evaluation
A facile, highly efficient, and greener method for the synthesis of new 1,4-disubstituted-1,2,3-triazoles was conducted using [Et3NH][OAc] as a medium by the implementation of ultrasound irradiation via click chemistry, affording excellent yields.
![Graphical abstract: New N-phenylacetamide-incorporated 1,2,3-triazoles: [Et3NH][OAc]-mediated efficient synthesis and biological evaluation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9RA03425K&imageInfo.ImageIdentifier.Year=2019)
RSC Adv., 2019,9, 22080-22091
https://doi.org/10.1039/C9RA03425K
Development of self-stratified antibacterial polymers via click chemistry
A new self-stratified contact-killing antimicrobial polyurethane was synthesized via efficient and orthogonal click-chemistry.
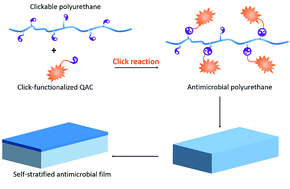
RSC Adv., 2019,9, 13159-13167
https://doi.org/10.1039/C9RA01572H
Synthesis of D-glyco-alkynone derivatives via carbonylative Sonogashira reaction
A carbonylative Sonogashira coupling approach to the synthesis of glyco-alkynones is described.
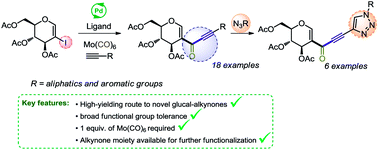
RSC Adv., 2019,9, 9468-9474
https://doi.org/10.1039/C9RA00523D
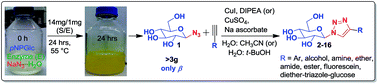
Gram scale production of 1-azido-β-D-glucose via enzyme catalysis for the synthesis of 1,2,3-triazole-glucosides
The retaining β-glucosidase acid/base mutant TxGH116D593A catalyzed the production of 1-azido-β-D-glucose for synthesis of 15 1,2,3-triazole β-glucosyl derivatives.
RSC Adv., 2019,9, 6211-6220
https://doi.org/10.1039/C9RA00736A
A new synthetic pathway based on one-pot sequential aza-Michael addition and photoCuAAC click reactions
A solvent-free process is described for the synthesis of tailor-made molecules from a one-pot, two-step approach combining aza-Michael addition and photoinduced copper(I) catalysed azide–alkyne (photo-CuAAC) reactions.

RSC Adv., 2019,9, 4824-4831
https://doi.org/10.1039/C8RA10011J
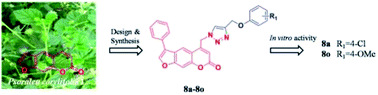
Preparation of novel 1,2,3-triazole furocoumarin derivatives via click chemistry and their anti-vitiligo activity
A novel 1,2,3-triazole furocoumarin derivatives with anti-vitiligo activity were synthesized via click chemistry.
RSC Adv., 2019,9, 1671-1678
https://doi.org/10.1039/C8RA09755K
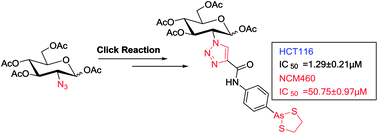
Carbohydrate-conjugated 4-(1,3,2-dithiarsolan-2-yl)aniline as a cytotoxic agent against colorectal cancer
We synthesized a carbohydrate-conjugated 4-(1,3,2-dithiarsolan-2-yl)aniline. It exhibited reduced cytotoxicity to normal cells, suggesting a feasible approach to improve the therapeutic index of arsenic-containing compounds as chemotherapeutic agents.
RSC Adv., 2018,8, 40760-40764
https://doi.org/10.1039/C8RA07860B
Photocatalytic copper-catalyzed azide–alkyne cycloaddition click reaction with Cu(II) coordination polymer
Cu(II) coordination polymers as photocatalysts for the copper-catalyzed azide–alkyne cycloaddition click reaction under household light irradiation in air.

RSC Adv., 2017,7, 52907-52913
https://doi.org/10.1039/C7RA10207K
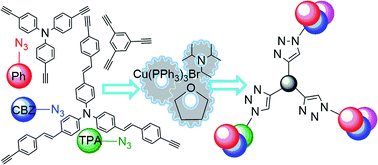
Efficient and straightforward click synthesis of structurally related dendritic triazoles
Structurally related triazolic dendrimers were efficiently synthesized applying CuAAC reaction.
RSC Adv., 2017,7, 47681-47688
https://doi.org/10.1039/C7RA09558A
Conversion of glycals into vicinal-1,2-diazides and 1,2-(or 2,1)-azidoacetates using hypervalent iodine reagents and Me3SiN3. Application in the synthesis of N-glycopeptides, pseudo-trisaccharides and an iminosugar
Glycals react with PIFA (or PIDA)–TMSN3 in presence of TMSOTf to form sugar derived 1,2-diazides and vicinal azidoacetates. Synthesis of 2-azido-N-glycopeptides, pseudotrisaccharides, and a piperidine triol derivative is reported.

RSC Adv., 2017,7, 41755-41762
https://doi.org/10.1039/C7RA08637G
Facile synthesis of novel hybrid POSS biomolecules via “Click” reactions
A novel alkyne-terminated cubic-octameric POSS was synthesised in high yield and click chemistry has been used to attach bio-oligomers.
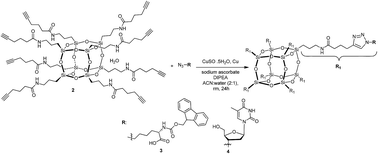
RSC Adv., 2017,7, 37474-37477
https://doi.org/10.1039/C7RA07915J
Continuous flow-ultrasonic synergy in click reactions for the synthesis of novel 1,2,3-triazolyl appended 4,5-unsaturated L-ascorbic acid derivatives
A combination of flow chemistry and batch-based synthetic procedures has been successfully applied to the assembly of novel 4,5-unsaturated L-ascorbic acid series 6a–6n with diverse C-6-substituted 1,2,3-triazole moiety.
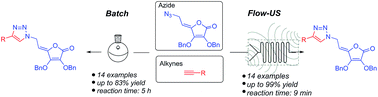
RSC Adv., 2017,7, 791-800
https://doi.org/10.1039/C6RA25244C
A recyclable and water soluble copper(I)-catalyst: one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles and their biological evaluation
A protocol for the synthesis of 1,4-disubstituted 1,2,3-triazoles via three-component reaction by a water soluble copper(I) complex has been developed.
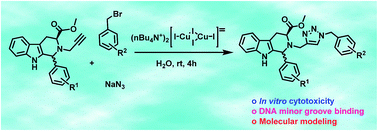
RSC Adv., 2016,6, 103556-103566
https://doi.org/10.1039/C6RA22942E
Copper-free click chemistry for microdroplet's W/O interface engineering
Microdroplets surface engineering using an azide fluorosurfactant prone to react with various functional heads conjugated beforehand to a strained alkyne.
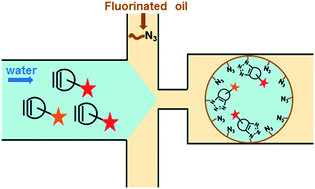
RSC Adv., 2016,6, 94942-94948
https://doi.org/10.1039/C6RA20385J
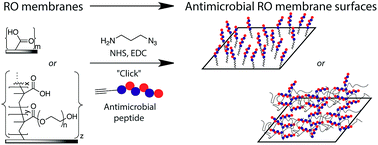
Attachment of antimicrobial peptides to reverse osmosis membranes by Cu(I)-catalyzed 1,3-dipolar alkyne–azide cycloaddition
Optimized polymer membrane surface modification with antimicrobial properties.
RSC Adv., 2016,6, 91815-91823
https://doi.org/10.1039/C6RA21930F
π-Stacking assisted redox active peptide–gallol conjugate: synthesis of a new generation of low-toxicity antimicrobial silver nanoparticles
We have synthesized, via click-chemistry, a redox-active peptide–gallol conjugate which facilitates rapid formation of antimicrobial silver nanoparticles with prominent antifungal activity.

RSC Adv., 2016,6, 85254-85260
https://doi.org/10.1039/C6RA13075E
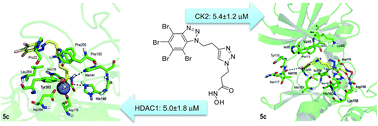
Design and synthesis of novel dual-target agents for HDAC1 and CK2 inhibition
Drug entities able to address multiple targets can be more effective than those directed to just one biological target.
RSC Adv., 2016,6, 66595-66608
https://doi.org/10.1039/C6RA09717K
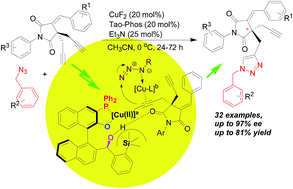
Tao-Phos-controlled desymmetrization of succinimide-based bisalkynes via asymmetric copper-catalyzed Huisgen alkyne–azide click cycloaddition: substrate scope and mechanism
The Cu(II)/Tao-Phos-catalyzed Huisgen cycloaddition of succinimide-derived bisalkynes resulted in chiral triazoles bearing a quaternary carbon-stereogenic center with good yields and chemoselectivity as well as moderate to high enantioselectivities (up to 97% ee).
RSC Adv., 2016,6, 58698-58708
https://doi.org/10.1039/C6RA13687G
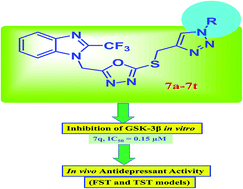
Synthesis of benzimidazole-based 1,3,4-oxadiazole-1,2,3-triazole conjugates as glycogen synthase kinase-3β inhibitors with antidepressant activity in in vivo models
Synthesized benzimidazole based 1,3,4-oxadiazole-1,2,3-triazole conjugates were found to inhibit GSK-3β activity in vitro and exhibit antidepressant-like activity in in vivo studies.
RSC Adv., 2016,6, 43345-43355
https://doi.org/10.1039/C6RA07273A
Design, synthesis and biological evaluation of novel triazole-core reversal agents against P-glycoprotein-mediated multidrug resistance
We designed and synthesized a novel series of P-glycoprotein (P-gp)-mediated multidrug resistance (MDR) inhibitors bearing a triazolphenethyl–tetrahydroisoquinoline scaffold through click chemistry.
RSC Adv., 2016,6, 25819-25828
https://doi.org/10.1039/C6RA02405J
Design, synthesis, biological evaluation and molecular docking of amide and sulfamide derivatives as Escherichia coli pyruvate dehydrogenase complex E1 inhibitors
Optimal binding mode of novel E. coli PDHc E1 inhibitor 9d.
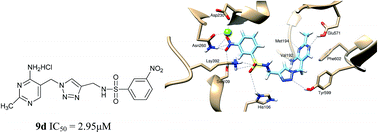
RSC Adv., 2016,6, 4310-4320
https://doi.org/10.1039/C5RA22573F
Facile preparation and properties of multifunctional polyacetylene via highly efficient click chemistry
A series of novel multifunctional polyacetylenes bearing oxadiazole and azo groups as molecular pendants were designed and synthesized via highly effective click chemistry.
RSC Adv., 2016,6, 448-455
https://doi.org/10.1039/C5RA21049F
Synthesis of novel 1,4-disubstituted 1,2,3-triazolo-bosentan derivatives – evaluation of antimicrobial and anticancer activities and molecular docking
RSC Adv., 2015,5, 105266-105278
https://doi.org/10.1039/C5RA18618H
A simple lateral flow biosensor for the rapid detection of copper(II) ions based on click chemistry
A simple and enzyme-free lateral flow biosensor for the rapid detection of Cu2+ based on copper(I) ion (Cu+)-catalyzed click chemistry has been constructed for the first time.
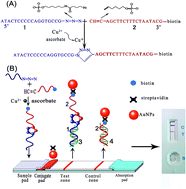
RSC Adv., 2015,5, 75722-75727
https://doi.org/10.1039/C5RA11752F
Click synthesis of a polyamidoamine dendrimer-based camptothecin prodrug
In the present work we report on the click synthesis of a new camptothecin (CPT) prodrug based on an anionic polyamidoamine (PAMAM) dendrimer intended for cancer therapy.
RSC Adv., 2015,5, 58600-58608
https://doi.org/10.1039/C5RA07987J
Dendron conjugation to graphene oxide using click chemistry for efficient gene delivery
Owing to its large surface area and rapid cellular uptake, graphene oxide (GO) is emerging as an attractive candidate material for delivery of drugs and genes.
RSC Adv., 2015,5, 50196-50211
https://doi.org/10.1039/C5RA07004J
2,4-Bis(4-aryl-1,2,3-triazol-1-yl)pyrrolo[2,3-d]pyrimidines: synthesis and tuning of optical properties by polar substituents
Novel D–π–A–π–D type chromophores – 2,4-bis(4-aryl-1,2,3-triazol-1-yl)pyrrolo[2,3-d]pyrimidines were prepared and their photophysical, electrochemical properties in conjunction with quantum chemical calculations were investigated.
![Graphical abstract: 2,4-Bis(4-aryl-1,2,3-triazol-1-yl)pyrrolo[2,3-d]pyrimidines: synthesis and tuning of optical properties by polar substituents](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5RA05482F&imageInfo.ImageIdentifier.Year=2015)
RSC Adv., 2015,5, 38610-38622
https://doi.org/10.1039/C5RA05482F
Highly efficient synthesis and characterization of multiarm and miktoarm star-long-branched polymers via click chemistry
Two series of 3–12 multiarm star polymers and 4-miktoarm star copolymer of butadiene and styrene, in which the Mn of arm was higher than 20 kg mol−1, were synthesized with high efficiency (from 85.0% to 96.1%) via click chemistry.

RSC Adv., 2015,5, 34466-34474
https://doi.org/10.1039/C5RA02168E
Functionalizable red emitting calcium sensor bearing a 1,4-triazole chelating moiety
Herein we developed a functionalizable OFF–ON red emitting fluorescent calcium probe based on a new chelating system formed by CuAAC click chemistry (Huisgen cycloaddition).
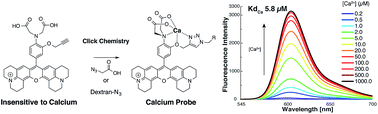
RSC Adv., 2015,5, 6993-7000
https://doi.org/10.1039/C4RA12858C
Development of a microfluidic “click chip” incorporating an immobilized Cu(I) catalyst
The development of a microfluidic “click chip” incorporating an immobilized Cu(I) catalyst for click reactions.
RSC Adv., 2015,5, 6142-6150
https://doi.org/10.1039/C4RA15507F
2-Pyrrolecarbaldiminato–Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature
2-Pyrrolecarbaldiminato–Cu(II) complexes were first established as efficient catalyst for 1,4-disubstituted 1,2,3-triazoles synthesis under green and mild reaction conditions.
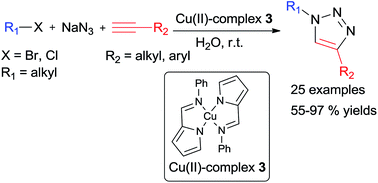
RSC Adv., 2015,5, 6661-6665
https://doi.org/10.1039/C4RA13423K
Highly efficient click reaction on water catalyzed by a ruthenium complex
Reactivity of ruthenium-catalyzed click reaction has been enhanced greatly by using H2O as the solvent.
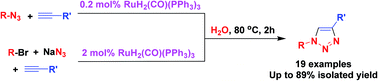
RSC Adv., 2015,5, 4693-4697
https://doi.org/10.1039/C4RA12960A
Copper immobilized onto a triazole functionalized magnetic nanoparticle: a robust magnetically recoverable catalyst for “click” reactions
A novel magnetic heterogeneous copper catalyst was synthesized by immobilization of copper ions onto triazole functionalized Fe3O4.
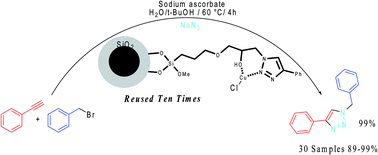
RSC Adv., 2015,5, 3894-3902
https://doi.org/10.1039/C4RA13330G
Click synthesis of graphene/poly(N-(2-hydroxypropyl) methacrylamide) nanocomposite via “grafting-onto” strategy at ambient temperature
Well-defined poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA) was grafted onto graphene sheets via click chemistry to afford a PHPMA/graphene nanocomposite with excellent dispersibility in organic solvents and water.
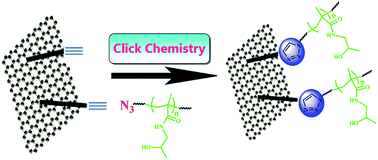
RSC Adv., 2014,4, 60920-60928
https://doi.org/10.1039/C4RA07825J
Photo-responsive reversible micelles based on azobenzene-modified poly(carbonate)s via azide–alkyne click chemistry
We provide a convenient method to construct photo-responsive poly(carbonate)s via ring-opening polymerization of cyclic carbonates followed by azide–alkyne click chemistry.
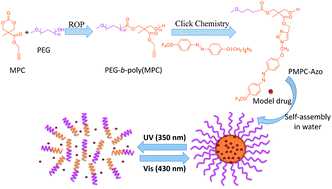
RSC Adv., 2014,4, 47929-47936
https://doi.org/10.1039/C4RA07345B
Modification of graphene oxide by a facile coprecipitation method and click chemistry for use as a drug carrier
A graphene oxide based ternary composite was synthesized for targeted drug carrier.
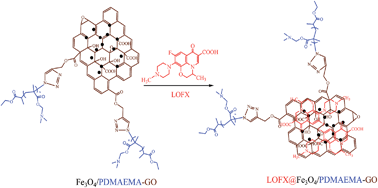
RSC Adv., 2014,4, 28807-28813
https://doi.org/10.1039/C4RA02219J
Synthesis and electrochemical properties of conducting polyaniline/graphene hybrids by click chemistry
Conducting polyaniline nanofiber-grafted graphene oxide (PANINF-GO) hybrids were prepared by click chemistry reaction and in situ rapid mixing polymerization. The resulting composites exhibited excellent electrochemical properties.
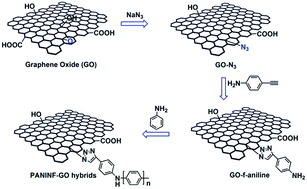
RSC Adv., 2014,4, 23936-23942
https://doi.org/10.1039/C4RA03275F
New clicked thiirane derivatives as gelatinase inhibitors: the relevance of the P1′ segment
A new family of triazolyl thiiranes has been synthesised and characterised as MMP-2 and MMP-9 inhibitors. The hit compound displayed submicromolar inhibition of MMP-2
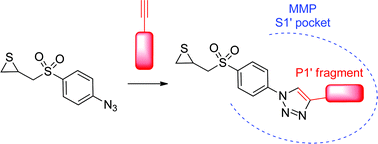
RSC Adv., 2014,4, 17726-17735
https://doi.org/10.1039/C3RA46402D
Chemoenzymatic synthesis of “click” xylosides and xylobiosides from lignocellulosic biomass
A range of triazole-linked O-xylosides and O-xylobiosides, with potential biological applications, has been prepared by an efficient two-step chemoenzymatic sequence from biomass-derived xylans.
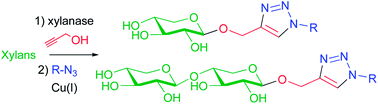
RSC Adv., 2014,4, 9330-9338
https://doi.org/10.1039/C3RA46173D
Copper-catalyzed one-pot synthesis of glycosylated iminocoumarins and 3-triazolyl-2-iminocoumarins
Efficient copper-catalyzed three-component synthesis of glycosylated iminocoumarins and 3-triazolyl-2-iminocoumarins.
RSC Adv., 2014,4, 5803-5814
https://doi.org/10.1039/C3RA46844E
Diversity oriented approach to triazole based peptidomimetics as mammalian sterile 20 kinase inhibitors
Post assembly peptide modifications – serine/threonine kinase inhibitors.
RSC Adv., 2013,3, 24447-24454
https://doi.org/10.1039/C3RA44318C
Capping of oligonucleotides with “clickable” m3G-CAPs
Methodology and synthesis of different m3G-CAP constructs equipped with an azide handle and their attachment by “click reaction” with an activated triple bond partner allows efficient formation of oligonucleotide m3G-CAP conjugates.
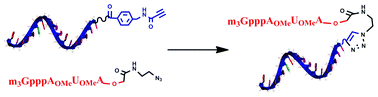
RSC Adv., 2012,2, 12949-12962
https://doi.org/10.1039/C2RA22345G
About this collection
Associate Editor Professor Manojit Pal (Dr Reddy’s Institute of Life Sciences, India) is the guest editor for this collection covering a decade of progress in click reactions based on CuAAC.
The Nobel Prize in Chemistry 2022 was awarded jointly to Professor Carolyn R. Bertozzi, Professor Morten Meldal and Professor K. Barry Sharpless for their seminal work in the development of click chemistry and bioorthogonal chemistry. Click chemistry has revolutionized the routes of molecular construction and has applications in drug discovery and development, medicinal and pharmaceutical chemistry, analytical chemistry, materials science, surface science, and more. Additionally, bioorthogonal chemistry has made it possible to monitor the chemical processes occurring in living cells, without interfering with native biochemical systems or causing cellular toxicity.
Click chemistry has not gone unnoticed over the years; many synthetic organic chemists have made contributions (both big and small) to this research area. So, in celebration of the Nobel Prize, we are delighted to launch a new collection comprising of relevant papers published in RSC Advances over last 10 years. The collection predominantly covers the application of click reactions in the areas of bioorganic and medicinal chemistry; papers devoted to the development of methodologies are also included.
RSC Advances welcomes new research papers and reviews focusing on the development and application of CuAAC based click reactions for further inclusion in the collection.
If you are interested in submitting to this collection, please contact the Editorial Office at advances-rsc@rsc.org