Synthesis of benzimidazole-based 1,3,4-oxadiazole-1,2,3-triazole conjugates as glycogen synthase kinase-3β inhibitors with antidepressant activity in in vivo models†
Abstract
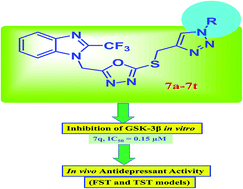
A series of novel benzimidazole-based 1,3,4-oxadiazole-1,2,3-triazole conjugates has been synthesized and evaluated for GSK-3β inhibitory activity in vitro. Compounds 7q, 7b, 7e and 7n exhibited significant inhibition with sub-micromolar IC50 values (0.15, 0.27, 0.32 and 0.39 μM respectively) and were examined further for antidepressant activity by forced swim test (FST) and tail suspension test (TST) models in Wistar rats. In both TST and FST models, all the test compounds were found to significantly reduce the immobility time period (displaying antidepressant-like activity) in comparison to a normal saline treated control group. Compound 7q was found to be the most active. Molecular docking studies of the active compounds 7q, 7b, 7e and 7n were also performed against GSK-3β to gain an understanding of their binding interactions.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...