1,2,3-Triazoles derived from olanzapine: their synthesis via an ultrasound assisted CuAAC method and evaluation as inhibitors of PDE4B†
Abstract
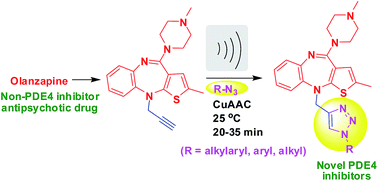
The antipsychotic drug olanzapine which does not inhibit PDE4 can be converted into the inhibitor of PDE4B via linking its N-10 position with an appropriately N-substituted 1,2,3-triazole moiety through a methylene linker. All these compounds were conveniently prepared by a CuAAC method under ultrasound irradiation at room temperature and evaluated for their PDE4 inhibitory potential in vitro. Three of them were identified as selective inhibitors of PDE4B (IC50 ∼ 5–6 μM) over PDE4D. Overall, the present research reports one of the few examples of an ultrasound assisted CuAAC method used in medicinal chemistry.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...