Quinoline-triazole hybrids inhibit falcipain-2 and arrest the development of Plasmodium falciparum at the trophozoite stage†
Abstract
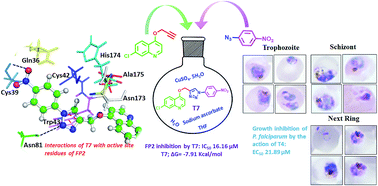
Falcipain-2 (FP2) is a papain family cysteine protease and a key member of the hemoglobin degradation pathway, a process that is required at erythrocytic stages of Plasmodium falciparum to obtain amino acids. In this study, we report a set of 10 quinoline-triazole-based compounds (T1–T10) which exhibit a good binding affinity for FP2, inhibit its catalytic activity at micromolar concentrations and thereby arrest the parasite growth. Compounds T4 and T7 inhibited FP2 with IC50 values of 16.16 μM and 25.64 μM respectively. Both the compounds T4 and T7 arrested the development of P. falciparum at the trophozoite stage with an EC50 value 21.89 μM and 49.88 μM. These compounds also showed morphological and food-vacuole abnormalities like E-64, a known inhibitor of FP2. Our results thus identify the quinoline-triazole-based compounds as a probable starting point for the design of FP2 inhibitors and they should be further investigated as potential antimalarial agents.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...