Click synthesis of a polyamidoamine dendrimer-based camptothecin prodrug†
Abstract
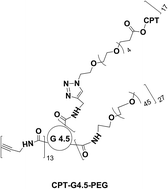
In the present work we report on the click synthesis of a new camptothecin (CPT) prodrug based on an anionic polyamidoamine (PAMAM) dendrimer intended for cancer therapy. We applied ‘click’ chemistry to improve the polymer-drug coupling reaction efficiency. Specifically, CPT was functionalized with a spacer, 1-azido-3,6,9,12,15-pentaoxaoctadecan-18-oic acid (APO), via a EDC/DMAP coupling reaction. In parallel, propargylamine (PPA) and methoxypoly(ethylene glycol) amine were conjugated to PAMAM dendrimer G4.5 in sequence using an effective coupling agent 4-(4,6-dimethoxy-(1,3,5)triazin-2-yl)-4-methyl-morpholinium chloride (DMTMM). CPT–APO was then coupled to the PEGylated PAMAM dendrimer G4.5–PPA via a click reaction using copper bromide/2,2′-bipyridine/dimethyl sulfoxide (catalyst/ligand/solvent). Human glioma U1242 cells were exposed to the CPT prodrug to determine the toxicity and cell cycle effects using a WST-1 assay and flow cytometry. The CPT prodrug displayed a dose-dependent toxicity with an IC50 of 5 µM, a 185-fold increase relative to free CPT, as a result of slow release. Furthermore, conjugated CPT resulted in G2/M arrest and cell death while the PEGylated dendrimer had little to no toxicity. Altogether, highly efficient click chemistry allows for the synthesis of multifunctional dendrimers for sustained drug delivery.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...