Chemoenzymatic synthesis of “click” xylosides and xylobiosides from lignocellulosic biomass
Abstract
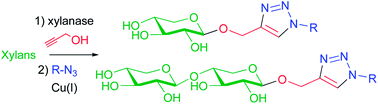
Synthesis of bio-based molecules from plant biomass with chemoenzymatic pathways represents a challenging task for the development of green chemistry. In this context, an efficient two-step chemoenzymatic sequence has been achieved for the preparation of triazole-linked xylosides and xylobiosides from biomass-derived xylans. The synthesis of propargyl xyloside and xylobioside catalysed by a xylanase in an aqueous medium was first studied and improved according to different reaction parameters. Cycloaddition reactions between the terminal alkyne moiety of these xylosides or xylobiosides and various aliphatic, aromatic or functionalized azides (“click chemistry”) afforded various triazole-linked O-xylosides and O-xylobiosides in high yields. These molecules are of interest for different biological applications.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...