Synthesis of d-glyco-alkynone derivatives via carbonylative Sonogashira reaction†
Abstract
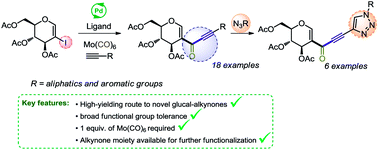
A carbonylative Sonogashira coupling approach to the synthesis of glyco-alkynones is described. Eighteen examples were obtained in moderate do nearly quantitative yields under mild conditions employing Mo(CO)6 as a safe carbon monoxide source. Functionalization of the alkynyl moiety via cycloaddition with organic azides provided six examples of glyco-triazoles.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC


 Please wait while we load your content...
Please wait while we load your content...