Functionalizable red emitting calcium sensor bearing a 1,4-triazole chelating moiety†
Abstract
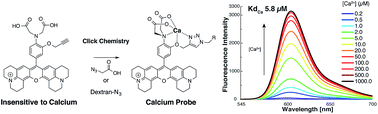
Herein we developed a functionalizable OFF–ON red emitting fluorescent calcium probe based on a new chelating system formed by CuAAC click chemistry (Huisgen cycloaddition). The pro-sensor 7 which is not sensitive to Ca2+, contains an alkyne moiety that, upon the click reaction, forms a chelating group involving the 1,4-triazole. Probe 10 exhibited good sensitivity towards calcium (Kd = 5.8 μM) and zinc (5.6 μM) with a high dynamic range (65 fold fluorescence increase), high quantum yield (0.59) and showed very low fluorescence enhancement in the presence of a high concentration of Mg2+. We extended this method and generated two dextran conjugates in order to compare their sensing properties with those of the molecular form of 10.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...