Diversity oriented approach to triazole based peptidomimetics as mammalian sterile 20 kinase inhibitors†
Abstract
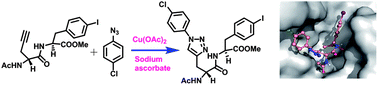
Protein kinases are important drug targets due to their involvement in several pathological conditions. Here, we demonstrate a strategy to develop small molecule inhibitors by using peptidomimetic compounds as a target for serine/threonine kinases. In this regard, we employ a unique approach to modify peptides by using a copper catalyzed 1,3-dipolar cycloaddition reaction between various azides and peptides containing an alkyne moiety to create a triazole based peptidomimetic library. Interestingly, these compounds have yielded competent kinase inhibitors, exhibiting an IC50 value of 1.2 μM for the pro-apoptotic mammalian sterile 20 1 (MST1) kinase, which is an important drug target in cardiomyopathy. Further, kinase profiling studies were performed with a panel of kinases both within the sterile 20 kinase family and also with PI-3K. Results revealed that compound 7 is a competent MST1 kinase inhibitor, which exhibits a high degree of selectivity and specificity for MST1 and does not inhibit the other tested kinases. In addition, docking studies shed light on the binding mode of these compounds. Hence, this knowledge can serve as a starting point for further development of triazole based peptidomimetics for designing selective serine/threonine kinase inhibitors.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...