A recyclable and water soluble copper(i)-catalyst: one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles and their biological evaluation†
Abstract
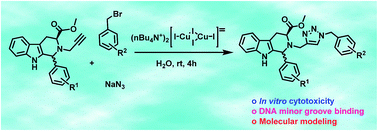
A facile protocol for the regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles via a three-component ‘click’ reaction of alkyl/benzyl bromides, sodium azide, and terminal alkynes catalyzed by an efficient water soluble copper(I) complex has been developed. The halides have been directly converted into 1,2,3-triazoles via in situ generation of azides and hence, handling of hazardous azide intermediates can be avoided. Gratifyingly, the inherent advantages of this one-pot process are the use of water as solvent, catalyst recyclability, reduced reaction times, simple recrystallization and high yields of the products. The broad scope of this protocol was also explored for the synthesis of a variety of diversely substituted biologically relevant DNA-interactive 1,2,3-triazolo-tetrahydro-β-carboline derivatives. These 1,2,3-triazolo-tetrahydro-β-carbolines were further evaluated for their in vitro cytotoxicity on selected human cancer cell lines of A-549, HCT-116, BT-549, MDA-MB-231, PC-3, NCI-H460 and HCT-15. DNA binding affinity through viscometry experiment and molecular modeling studies have indicated efficient minor groove binding of these new scaffolds.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...