Tao-Phos-controlled desymmetrization of succinimide-based bisalkynes via asymmetric copper-catalyzed Huisgen alkyne–azide click cycloaddition: substrate scope and mechanism†
Abstract
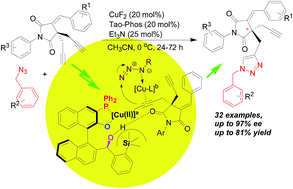
It was found that the catalytic Huisgen cycloaddition reaction of succinimide-derived bisalkynes with azides resulted in useful succinimide-derived triazoles bearing quaternary carbon-stereogenic centers with good to excellent yields as well as good chemoselectivity and moderate to high enantioselectivities (up to 97% ee), in which the CuF2/Tao-Phos complex was proved to be an effective bimetallic catalyst in the presence of triethylamine. The mechanistic studies based on the effect of reaction parameters on stereoselectivity as well as ESI-MS analysis suggested that our ligand (Tao-Phos) and related binuclear copper centers play a crucial role in this asymmetric click chemistry because of the strong catalyst–substrate interaction with the aid of a possibly in situ formed binuclear or multinuclear copper-based transition state. This work not only provides a new example of a Cu(II)-mediated asymmetric AAC reaction involving chelating base and azides, but also suggests the significance of binuclear copper species in catalytic asymmetric click reactions with two copper center-involved enantioselective inductions.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...