Open Access Article
Open Access ArticleDesign, synthesis, biological evaluation and molecular docking study of novel pyridoxine–triazoles as anti-Alzheimer's agents†
Tiyas Pala,
Saipriyanka Bhimanenib,
Abha Sharma *a and
S. J. S. Flora
*a and
S. J. S. Flora *b
*b
aDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Raebareli, India. E-mail: abha.sharma@niperraebareli.edu.in
bDepartment of Regulatory Toxicology, National Institute of Pharmaceutical Education and Research, Raebareli, India. E-mail: sjsflora@hotmail.com
First published on 9th July 2020
Abstract
A series of multi-target natural product-pyridoxine based derivatives were designed, synthesized, characterized and evaluated as anti-Alzheimer agents. In vitro testing revealed the multi-functional properties of compounds such as inhibition of acetylcholinesterase (AChE), antioxidant and metal chelation. Among the series, 5i derivative was found most potent AChE inhibitor, possess antioxidant potential and chelating metal ions. Further binding interaction of 5i with AChE was studied using molecular docking, showed interaction with both PAS and CAS site of AChE. In silico predictions were also performed to predict toxicity and ADME properties of the molecule 5i and found within drug likeness range. Therefore, 5i could be a promising multi-functional compound that can be used for further development of novel drug for Alzheimer disease.
1 Introduction
Alzheimer's disease (AD) is the most challenging neurodegenerative disease affecting mostly the population above 65 years of age. The perplexing nature of the disease makes it all more difficult for its ministration. More than 50 million are suffering globally and the number is likely to get hiked up to 150 million by the year 2050.1 The multifaceted nature of the disease with several interconnected etiologies led to the idea of developing multi-target directed ligands (MTDLs). MTDLs in the field of neurodegenerative diseases is now a very significant area of research developed by academia and industry.2,3 The design of MTDL comprises two or more pharmacophore moieties which are responsible for targeting multiple targets which are accountable for the pathogenesis of the disease. Therefore, MTDLs are single molecules with multiple functions which are strategically designed to serve the purpose of developing potential new chemical entities that can be used against AD.The currently available Food and Drug Administration (FDA) approved drugs mainly focus on the cholinergic hypothesis, as one of the most excavated areas to slow down the progression of AD. Cholinergic hypothesis, amyloid-β cascade hypothesis, metal dyshomeostasis, tau hypothesis and oxidative stress hypothesis are some of the identified etiologies responsible for this disease. Damage to cholinergic neurons lead to cognitive decline which showed the way to develop acetylcholinesterase (AChE) inhibitors providing symptomatic relief to the AD patients. However, this age related disease poses a problem of uncontrollable bio-burden of metal ions in the brain. This metal dyshomeostasis in turn induces oxidative stress and formation of amyloid-β (Aβ) plaques. Due to this interconnection of etiologies, classical metal chelator cannot do any benefit to this existing problem of the disease. Therefore MTDLs is needed in this juncture. Increase in bio-metals amplifies reactive oxygen species (ROS) which includes augmentation of lipid peroxidation, protein oxidation, nitration and glycol oxidation. Oxidative stress aggravates the pathophysiological conditions of neuronal damage.4 A molecule having additional anti-oxidant potency along with AChE inhibitory potential and metal chelating ability can be developed as a potential anti-AD agent. Studies have stated that peripheral anionic site (PAS) binding of AChE may promote Aβ aggregation. For PAS binding, aromatic rings with pi–pi stacking are recommended as derived from the docking studies with donepezil.5
In light of the concepts mentioned above, and to focus on our efforts to develop a new class of anti-Alzheimer's agents as MTDLs, we focused on the synthesis of pyridoxine based 1,2,3-triazoles and its biological evaluation. We assessed the synthesized compounds for AChE inhibition, antioxidant potency, metal chelation ability using in vitro assays as screening methods. We predicted various pharmacokinetic properties and toxicity of the best compound of the series using in silico tools.
2 Results and discussion
2.1 Design
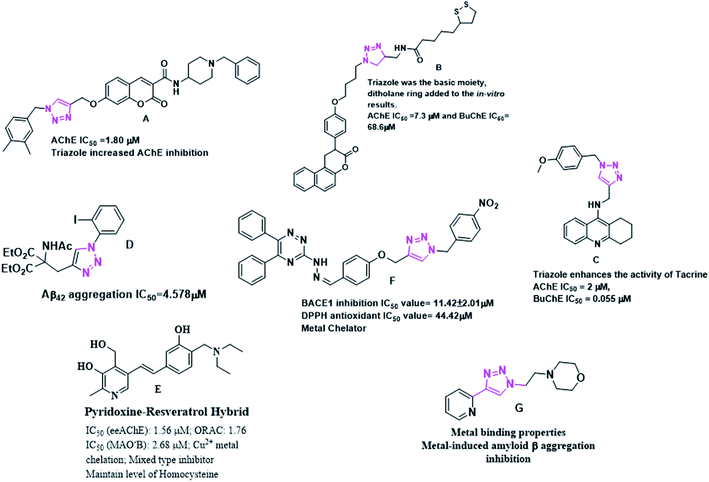
Nitrogen heterocycles have long been of medical interest6–8 while, triazoles falling under the category of nitrogen heterocycles have been the moiety of interest. Numerous molecules with triazole moiety have been reported. In this regard, Rastegari et al. reported 1,2,3-triazole chromenone carboxamide derivatives as AChE inhibitors.9 Jalili-Baleh et al. developed 3-phenylcoumarin lipoic acid based triazole derivatives as anti-Alzheimer's agents.10 Fig. 1 provides a summary of few triazole and pyridoxine containing molecules reported recently as potential anti-Alzheimer's agents.9–15Pyridoxine is known to reduce homocysteine levels in Alzheimer's patients. Homocysteine is derived from amino acid methionine and is a non-protein homologue of cysteine. The directly attached hydroxyl group to the pyridine nucleus might be playing a role in chelation of metal and also for the anti-oxidant properties. Pyridoxine has successfully completed the phase 3 clinical trials as a vitamin which can reduce homocysteine levels in AD affected patients. The circulating high level of homocysteine in AD is responsible for deposition of amyloid β plaques.16,17 Pyridoxine–resveratrol have been reported as only pyridoxine based compounds against AD. However, pyridoxine based triazoles have not been reported and therefore, herein we report this new class of compounds.
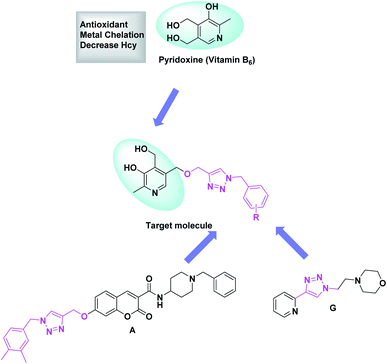
Taking into the consideration the multifaceted nature of the disease, we designed pyridoxine based 1,2,3-triazoles as an MTDL which might be an effective agents against AD. We have designed our target molecules containing both the pyridoxine and triazole moiety as a new class of MTDL using linking approach. Fig. 2 provides the possible hypothesis behind the designing of our target molecules.
2.2 Chemistry
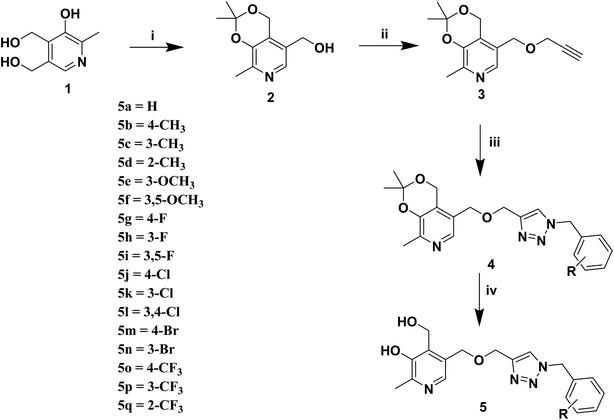
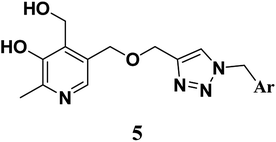
We synthesized pyridoxine based triazoles derivatives (Scheme 1). The target molecules were synthesized via four step reaction scheme using pyridoxine hydrochloride (1) as starting material. In the first step, commercially available pyridoxine hydrochloride (1) was protected at the 3- and 4-hydroxy groups using p-toluenesulfonic acid monohydrate and dry acetone in the presence of 2,2-dimethoxypropane under argon atmosphere. Then compound 2 was treated with propargyl bromide using NaH as base and dry THF as solvent under argon atmosphere to give compound 3. In the next step, various substituted benzyl azides were generated in situ from benzyl bromides followed by synthesis of triazole moiety after addition of compound 3 using the classical CuAAC, generating compounds 4(a–q). Finally, target compounds 5(a–q) were obtained through deprotection of the isopropylidene group.2.3 In vitro assay of AChE inhibition
The in vitro activity of the new synthesized molecules was done using AChE from electric eel (EeAChE) following Ellman's method.25 All the molecules were tested for their ability to inhibit AChE activity, keeping donepezil as the reference compound. The IC50 values of the molecules are summarized in Table 1. AChE inhibition remains a very critical end point in slowing down the progression of the disease, and thus we selected to determine the ability of our compounds to inhibit the aforesaid enzyme.| Compound | Ar | EeAChE IC50 ± SEMa (mM) | ORAC index ± SEMb | ||
|---|---|---|---|---|---|
| a IC50 values is the concentration of the enzyme required to decrease the enzyme activity by 50% and are the mean of three independent experiments, represented in mean ± SEM (SEM = standard error mean).b The mean ± SEM of three independent experiments. Data are represented as ORAC-FL values of Trolox equivalents (μM of tested compound/μM of Trolox).c n.a = not active.d N.T = not tested. | |||||
| 5a |  |
>10 | 1.0564 | ±0.3604 | |
| 5b | 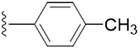 |
3.3833 | ±0.0927 | 1.8428 | ±0.0980 |
| 5c | 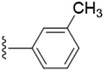 |
1.3759 | ±0.1019 | 2.0164 | ±0.0538 |
| 5d | 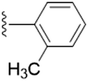 |
0.9073 | ±0.0924 | 1.9723 | ±0.4236 |
| 5e | 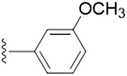 |
4.5737 | ±0.0995 | 1.8862 | ±0.2533 |
| 5f | 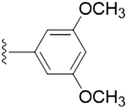 |
2.6508 | ±0.1694 | 2.3425 | ±0.0228 |
| 5g | 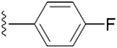 |
6.4687 | ±0.1334 | 1.4489 | ±0.1579 |
| 5h | 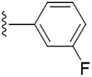 |
1.5437 | ±0.0227 | 2.1015 | ±0.0315 |
| 5i | 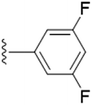 |
1.5609 | ±0.0237 | 1.2138 | ±0.2832 |
| 5j | 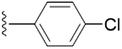 |
2.7187 | ±0.0435 | 1.9697 | ±0.1800 |
| 5k | 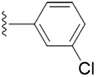 |
2.70723333 | ±0.0127 | 0.8358 | ±0.0374 |
| 5l | 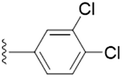 |
3.3777 | ±0.2162 | 1.9421 | ±0.1603 |
| 5m | 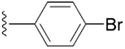 |
3.1383 | ±0.0432 | 1.215 | ±0.1383 |
| 5n | 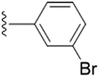 |
1.0801 | ±0.0851 | 2.2079 | ±0.1003 |
| 5o | 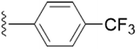 |
6.5162 | ±0.1326 | 2.0758 | ±0.1775 |
| 5p | 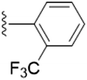 |
3.6525 | ±0.0120 | 0.8718 | ±0.2761 |
| 5q | 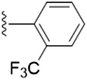 |
1.1346 | ±0.1632 | 0.7168 | ±0.1410 |
| Pyridoxine | — | n.ac | 0.5249 | ±0.1084 | |
| Donepezil | — | <5 μM | N.Td | ||
| Trolox | — | N.Td | 1 | ||
The unsubstituted 5a did not show much inhibition even at the highest concentration (10 mM), thus can be considered inactive towards the enzyme. With the substitution of methyl group at the para position (5b) it however, showed inhibitory activity as compared to 5a. By changing the position of methyl group from para to meta (5c) and ortho (5d) position, a marked change was noted. A meta substitution of the electron donating group (EDG) offered a better inhibitory activity almost twice potent as compared to 5b, while the ortho alkyl substitution proved even better. Substitution of methoxy group at the 3rd position (5e) and at 3rd and 5th position of the aromatic ring (5f) had no inhibitory activity, however, methyl substitution proved better than these. A double methoxy substitution was found more efficacious compared with its single substitution.
We further explored by trying different halides as substituents and their effect on the inhibition on the enzyme. We also synthesized F, Cl and Br derivatives at different position. Halogens are supposed to have +R effect on the aromatic ring, despite of their position. When the effect of halogen is observed at the para position, fluoro substituent (5g) showed lesser efficacy compared to –Cl (5j) and –Br (5m) derivatives. However, we did not find a uniform trend in activity as we moved down the periodic table. Chloro substitution at the para position was more potent in comparison to bromo substitution. However, meta substitution of halogens showed much better results as compared to the para substitution. Change in position from para to meta did not prove successful in improving potency in chloro substitution (5k), as there was no such change in the IC50 value. Bromine at the 3rd position of the aromatic ring (5n) was more active in comparison to the fluoro substitution (5h). We even tried to find out the effect of substitution when two positions on the aromatic ring are occupied with the halogen atoms. We thus included fluorine in 3rd and 5th position (5i) and in another compound, chlorine at 3rd and 4th position (5l). Inclusion of two fluorine atoms at both the meta positions, did not show change in potency but there was a spurt in % of enzyme inhibition. To summarize the effect of di-substitution on the aromatic ring, 5i was considered the best as compared to 5l and 5f. Owing to the fact that fluorine and methoxy groups are both ring activators, the difference in potency and percentage of inhibition between the two can be due to the high electronegativity and small size of fluorine.
We tried with trifluoro-methyl substitution at para, meta and ortho positions (5o, 5p, 5q). Trifluoro-methyl substitution at the ortho position was the best amongst the three both from the perspective of potency and inhibitory activity. However, when compared with methyl substitution, 5b was double potent in respect to 5o. We got the same observation for meta substitution as well, 5c was twice in terms of potency as compared to 5p. Methyl substitution at the ortho position was marginally better in terms of trifluoro-methyl substitution at the same position. Methyl group is considered as ring activator, while trifluoro-methyl group as ring deactivator. Therefore, the observations confirm that EDG is best suited for inhibitory activity against AChE, when pyridoxine based triazoles are concerned. Table 2 gives a detailed information of percentage of inhibition of AChE at different concentrations of by 5c, 5d, 5h, 5i, 5n, 5q and the reference compound, donepezil. Fig. 3 shows the comparative results of percentage of inhibition of AChE by 5c, 5d, 5h, 5i, 5n, 5q derivatives at 10 mM concentration, taking donepezil as the reference standard.
| 156 μM | 312 μM | 625 μM | 1.25 mM | 2.5 mM | 5 mM | 10 mM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ±SEM | % | ±SEM | % | ±SEM | % | ±SEM | % | ±SEM | % | ±SEM | % | ±SEM | |
| a Data presented here is of % of AChE inhibition mean ± SEM of three independent experiments at different concentrations. Donepezil is taken as the reference compound. | ||||||||||||||
| 5c | 22.49 | 3.62 | 26.92 | 0.55 | 39.11 | 2.48 | 53.8 | 3.13 | 64.11 | 1.79 | 73.43 | 1.13 | 81.92 | 0.85 |
| 5d | 33.53 | 6.44 | 36.41 | 6.8 | 51.15 | 5.55 | 58.56 | 4 | 69.57 | 2.3 | 81.3 | 2.36 | 86.03 | 0.63 |
| 5h | 19.04 | 0.29 | 25.49 | 2.22 | 35.1 | 1.59 | 56.37 | 1.85 | 60.48 | 1.24 | 66.56 | 0.26 | 69.02 | 0.36 |
| 5i | 14.97 | 4.12 | 26.58 | 3.92 | 35.38 | 0.47 | 43 | 1.52 | 61.41 | 2.82 | 66.78 | 0.1 | 77.89 | 0.15 |
| 5n | 14.69 | 1.15 | 25.79 | 2.73 | 41.99 | 1.42 | 57.39 | 4.99 | 70.16 | 4.11 | 75.88 | 2.44 | 86.28 | 1.38 |
| 5q | 22.49 | 3.62 | 26.92 | 0.55 | 39.11 | 2.48 | 53.8 | 3.13 | 64.11 | 1.79 | 73.43 | 1.13 | 81.92 | 0.85 |
| Donepezil | 89.07 | 1.19 | 94.5 | 0.81 | 97.44 | 0.73 | 100.88 | 0.93 | 99.7 | 1.04 | 99.74 | 0.69 | 99.95 | 0.48 |
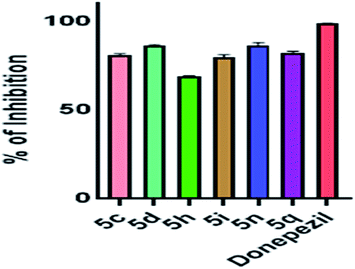 | ||
| Fig. 3 Percentage of inhibition of AChE by 5c, 5d, 5h, 5i, 5n, 5q derivatives at 10 mM concentration. Data presented here is mean ± SEM of three independent experiments. | ||
To summarize all our observations with respect to our standard compound donepezil, our molecules required a higher concentration to show their inhibitory activity. Donepezil showed almost 89% inhibition at the highest dilution in which we tested our compounds (156 μM) and 65% inhibition at 5 μM. However, at the highest concentration, that is, at 10 mM concentration, compounds showed a maximum of 86% inhibitory activity towards AChE while donepezil exhibited 100% activity.
2.4 Antioxidant study
Aging is associated with oxidative stress resulting in generation of reactive oxygen species. We determined the oxygen radical absorption capacity-fluoroscein (ORAC-FL) values for the entire series. The ORAC-FL values are given in Table 1 and are a measure of the total antioxidant capacity. We generated peroxyl free radicals using the free radical generator 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) and fluorescein as the fluorescent probe in this assay at 37 °C. The total antioxidant capacity of the molecules were determined in equivalents to Trolox (vitamin E analogue), keeping both at same concentration (8 μM). One of the reasons for choosing pyridoxine as a pharmacophore was for it's antioxidant nature. We observed all our compounds to have anti-oxidant property as shown in Table 1.Vitamin B6 being a dietary supplement was tested using the ORAC-FL method. All the compounds showed almost similar anti-oxidant nature, however, pyridoxine showed the most efficacy in terms of an anti-oxidant. Pyridoxine was taken as a reference compound to evaluate the entire series in this experiment.
Therefore, from the observations of Table 1, we selected 5c, 5d, 5h, 5i, 5n and 5q on the basis of AChE inhibitory activity and antioxidant property of the compounds for further evaluations.
2.5 Metal chelation study
Chelation of bio-metals will make the compounds prove as MTDLs and be an added advantage. We tested 5c, 5d, 5h, 5i, 5n and 5q against bio-metals Fe3+, Cu2+, Zn2+, Al3+ which are responsible for AD, using UV spectrophotometer within the wavelength range of 200 to 600 nm. All these bio-metals are responsible for the pathophysiological conditions in AD. We found a marked ability within the compounds to chelate Fe3+. Compounds 5c, 5i and 5q chelated both Fe3+ and Al3+, 5i chelated the metals most with a 222% increase in absorbance at 292 nm which also includes a bathochromic shift of about 3 nm in case of Fe3+ and an increase of 104% in absorbance at 292 nm which too included a bathochromic shift of 4 nm in case of Al3+. The observations for all the compounds studied for metal chelation a study has been tabulated in Table 3.| Compound | Cu2+ | Zn2+ | Al3+ | Fe3+ | |||||
|---|---|---|---|---|---|---|---|---|---|
| % ΔAa | Shiftb | % ΔAa | Shiftb | % ΔAa | Shiftb | % ΔAa | Shiftb | ||
| a Data represented here is percentage change in absorbance; (+) indicating a decrease in absorbance and (−) as increase; calculated by the formula [(abs of tested compound − abs of complex)/abs of tested compound] × 100.b Data represented here is shift in nm; (+) indicates a hypsochromic shift and (−) indicates a bathochromic shift as calculated from the formula [λmax of tested compound − λmax of complex]. | |||||||||
| 5c | |||||||||
| λmax | 256.39 | 0 | 0 | 0 | 0 | — | — | — | — |
| 287.7 | 0 | 0 | 0 | 0 | (+)58.41 | (−)4.76 | (−)171.14 | (−)6.22 | |
| 326.31 | 0 | 0 | 0 | 0 | (+)141.5 | (+)0.42 | — | — | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 5d | |||||||||
| λmax | 254.28 | (+)8.76 | (−)1.21 | (+)6.04 | (−)1.21 | (+)9.36 | (−)1.21 | (+)48.04 | 0 |
| 288 | (−)34.63 | 0 | (−)34.62 | (−)1 | (−)31.94 | (−)2 | (−)1.19 | (−)4 | |
| 324.35 | (+)3.75 | (+)0.79 | (−)8.41 | 0 | (−)7.13 | (−)0.41 | (−)21.00 | 0 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 5h | |||||||||
| λmax | 257.15 | (−)10.20 | (+)1.03 | (+)17.14 | (+)0.31 | (+)11.02 | 0 | (−)65.31 | (+)0.67 |
| 288.5 | (−)9.15 | (−)0.5 | (+)20.42 | (−)0.36 | (+)3.52 | (+)1.4 | (−)105.63 | (+)0.67 | |
| 325.74 | (+)5.65 | (+)0.74 | (+)11.01 | (−)0.26 | (+)10.42 | (−)0.26 | (−)27.08 | (+)0.36 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 5i | |||||||||
| λmax | 257.85 | 0 | 0 | 0 | 0 | — | — | — | — |
| 288.78 | 0 | 0 | 0 | 0 | (−)104.57 | (−)3.68 | (−)221.83 | (−)2.6 | |
| 325.93 | 0 | 0 | 0 | 0 | (+)43.01 | (−)1.09 | (+)48.38 | (−)3.25 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 5n | |||||||||
| λmax | 254.72 | (+)17.34 | (−)1.09 | (+)1.24 | (−)1.08 | (+)5.88 | (−)1.08 | (−)54.80 | (−)0.72 |
| 290.57 | (+)18.93 | (+)0.67 | (+)2.43 | (+)2.07 | (+)5.82 | (+)2.07 | (−)70.39 | (+)2.07 | |
| 324.71 | (+)16.59 | 0 | (+)4.255 | (−)0.67 | (+)4.25 | (−)0.67 | (−)23.62 | (+)0.36 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 5q | |||||||||
| λmax | 258.23 | 0 | 0 | 0 | 0 | — | — | — | — |
| 288.46 | 0 | 0 | 0 | 0 | (−)78.07 | (−)3.62 | (−)185.50 | (−)2.92 | |
| 325.56 | 0 | 0 | 0 | 0 | (+)39.70 | 0 | (+)47.94 | 0 | |
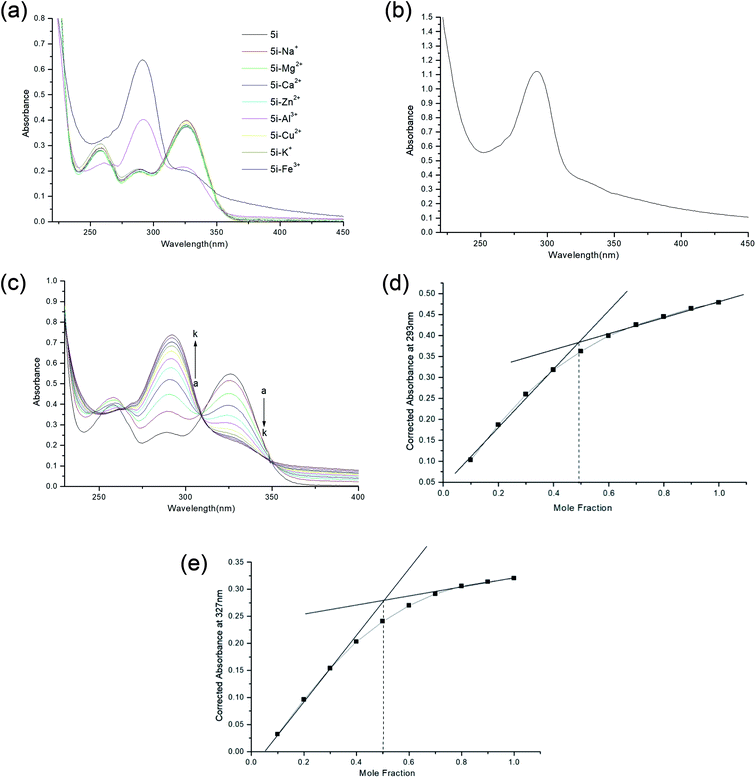
Although, aluminium being the most abundant metal on the earth's crust, it do not pose an immediate health concern when being exposed to the human body. Chronic exposure to aluminium in drinking water has been studied for it's association with AD. To add more to the pathophysiology, aluminium is known to enhance ROS generation and stimulated iron based generation of ROS species. Both these metals are also known to be associated with the senile plaques.19 Therefore, 5i has the ability to chelate both the trivalent cations which can add well to the property of the compound as a MTDL as shown in Fig. 4a.
The main challenge for chelation therapy of metals is selectivity towards metal cations. So we sought to check the selectivity towards essential metals responsible for proper physiological functions of our body. We included Ca2+, Mg2+, Na+ and K+ in our study and interestingly found out that 5i did not chelate these metal ions at all (Fig. 4a). The spectrum of 5i–Fe3+ complex was quite different from the parent spectrum and those by other metals. Owing to the fact, that our biological system comprise a mixture of metal ions, we sought to check the affinity of our compound 5i towards Fe3+ in the presence of other metals (Fe3+, Zn2+, Cu2+, Ca2+, Mg2+, Na+ and K+) present in equal amount. However, we excluded Al3+ from the study as the purpose was to check affinity towards Fe3+ and Al3+ does not form an essential part of our system. We observed the characteristic curve of 5i–Fe3+ as 5i showed affinity towards Fe3+ in the metal pool (Fig. 4b).
We studied the stoichiometric association of the Fe3+–5i complex by keeping the amount of ligand fixed and titrating the amount of metal in increasing concentration ranging from 0–100 μM using UV spectrophotometer. The spectral changes were recorded at nm and a graph was plotted (Fig. 4c). There was an increase in intensity at 293 nm and a decrease at 327 nm, on successive increase in concentration of Fe3+. We analysed the data and plotted the Job's plot, at 239 nm and 327 nm where on extrapolation we got a mole fraction of 0.5 in both the wavelength confirming formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex between 5i and Fe3+ (Fig. 4d and e).
1 stoichiometric complex between 5i and Fe3+ (Fig. 4d and e).
Good et al. performed a laser microprobe study or the LAMMA study where it was found selective deposition of Al and Fe in the neurofibrillary tangles.20 Therefore, 5i being a selective aluminium and iron chelator can be a potential inhibitor against these potentially toxic metals and can surely halt the mechanism of pathological important structures.
2.6 In silico study
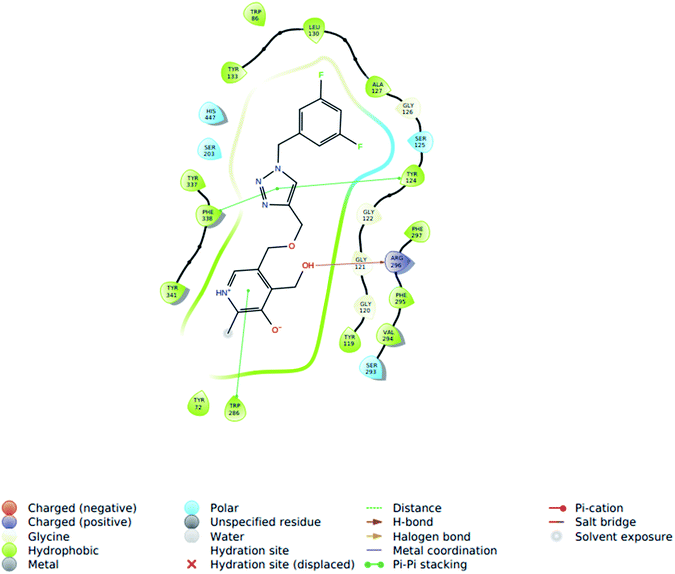 | ||
| Fig. 5 2D representation of molecular docking study of 5i with AChE (PDB ID 4EY5). | ||
The ligand showed interactions with the PAS site amino acids of AChE and binding to the PAS site of the enzyme is known to inhibit Aβ aggregation due to the homologous domains of the β-neurexin with PAS site, itself serving as a ligand. Thus in silico results of molecular docking with ligand validated the results obtained from the in vitro AChE experiments.
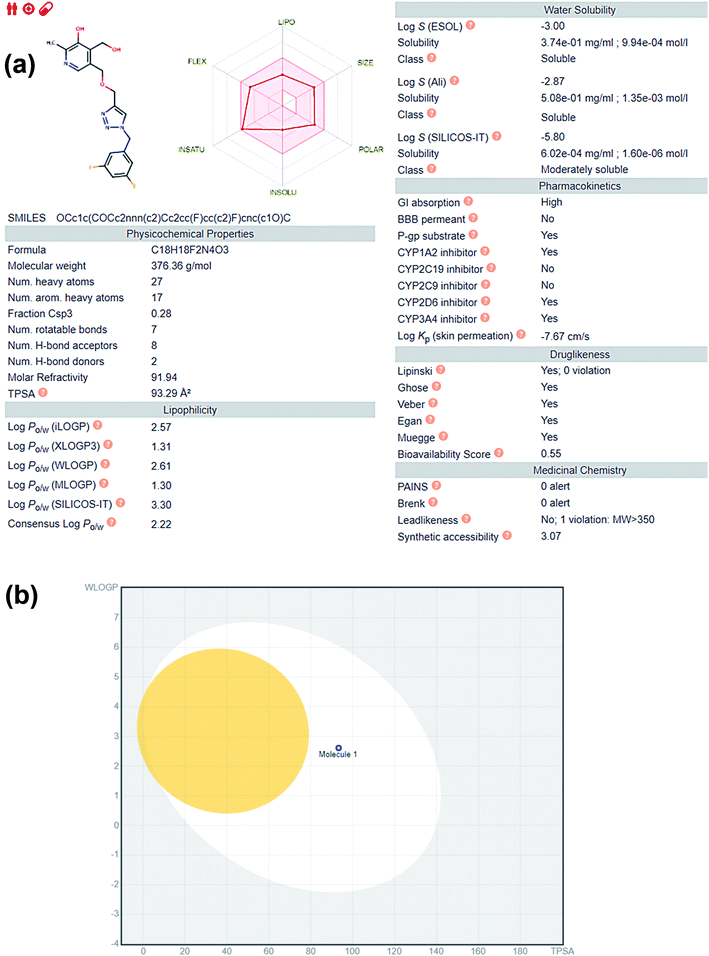
Fig. 6b is the BOILED EGG representation of the permeation properties of the molecule 5i. The figure displays two parts, the white part representing those set of values which allows gastric permeation and the yolk or the yellow region representing those set of values which allows blood–brain-barrier permeation. The graph is plotted against W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P[a
P[a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P(n-octanol/water partition coefficient) method developed by Wildman and Crippen] versus TPSA or Topological Polar Surface Area in the BOILED EGG representation. The blue dot in the figure written as Molecule 1 is of 5i.
P(n-octanol/water partition coefficient) method developed by Wildman and Crippen] versus TPSA or Topological Polar Surface Area in the BOILED EGG representation. The blue dot in the figure written as Molecule 1 is of 5i.
3 Conclusion
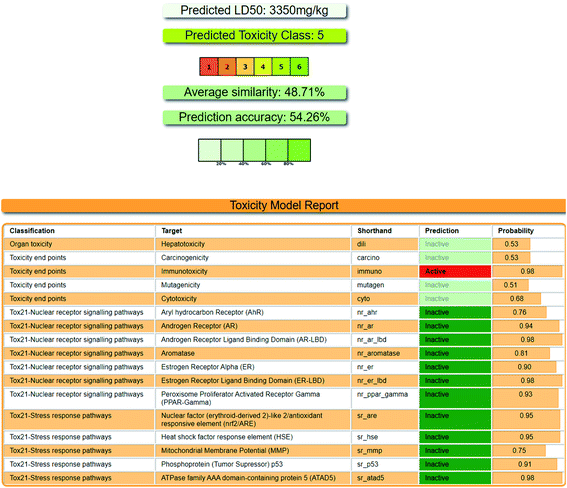
Here, we report the design, synthesis and biological evaluations of a novel class of pyridoxine based triazole as multi-target directed ligands. Pyridoxine itself is very crucial for the homocysteine balance in AD patients and as well as considered an important vitamin for our daily diet. However, as proved from our experiment, it is not an AChE inhibitor. Out of the seventeen synthesized pyridoxine based triazoles, six showed good AChE inhibition and antioxidant potency. Meta and ortho substitution on the aromatic ring with EDG or ring activator proved beneficiary for AChE activity. With further metal chelation studies, we found 5i as the best compound of this new series. 5i has good AChE inhibitory activity (IC50 = 1.56 ± 0.02 mM) with 77.89% of inhibition at highest concentration and possessed antioxidant property having ORAC-FL value of 1.21 ± 0.28 equivalent to Trolox, whose ORAC-FL value is taken as 1. The in vitro results for 5i has been validated with the help of molecular docking with AChE by investigating it's binding poses. The compound 5i chelated Fe3+ as the spectra showed about 220% increase in absorbance and a 104% increase was seen in case of Al3+, exhibiting bathochromic shift in both. Selectivity towards targeted metal ion has always been a challenge in metal chelation therapeutics, henceforth, further throwing light on the ability of 5i to selectively chelate Fe3+ from a pool of metal ions, gives it an added advantage. None of the literature of triazole based anti-Alzheimer's agents have reported selectivity towards metal ions with a drastic change in absorbance. We concluded our work with in silico studies of drug likeness, pharmacokinetic properties of 5i, along with toxicity predictions which predicted it to be under Class 5 according to the toxicity labelling of chemicals. Our initial findings can open up new therapeutic areas in multi-target directed ligands against AD.4 Experimental section
4.1 Chemistry
4.1.2.1 (2,2,8-Trimethyl-4H-[1,3]dioxino[4,5-c]pyridin-5-yl)methanol (2). White solid crystals; mp-110–113 °C; Rf = 0.5 (ethyl acetate/hexane 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); 1H NMR (500 MHz, chloroform-d) δ 7.77 (s, 1H, N
2); 1H NMR (500 MHz, chloroform-d) δ 7.77 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 4.91 (s, 2H, O–C
) δ 4.91 (s, 2H, O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.52 (s, 2H, C
2) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 2.34 (s, 3H, C
2–OH) δ 2.34 (s, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) δ 1.53 (s, 6H, 2 × C
3) δ 1.53 (s, 6H, 2 × C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 145.44, 145.09, 138.42, 130.59, 125.24, 99.41, 58.24, 57.88, 24.53, 18.24; IR (cm−1) ν: 3851.17, 3619.70, 1695.98, 1213.27.
3); 13C NMR (125 MHz, chloroform-d) δ 145.44, 145.09, 138.42, 130.59, 125.24, 99.41, 58.24, 57.88, 24.53, 18.24; IR (cm−1) ν: 3851.17, 3619.70, 1695.98, 1213.27.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 ratio of hexane–DCM solvent system to provide compound 3 in 75% yield.
80 ratio of hexane–DCM solvent system to provide compound 3 in 75% yield.
4.1.3.1 2,2,8-Trimethyl-5-((prop-2-yn-1-yloxy)methyl)-4H-[1,3]dioxino[4,5-c]pyridine (3). Brown oil; Rf = 0.6 (DCM/methanol 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.99 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.99 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 4.9 (s, 2H, O–C
) δ 4.9 (s, 2H, O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.71 (s, 2H, C
2) δ 4.71 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.46 (s, 2H, C
2–OH) δ 4.46 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C) δ 2.4 (s, 3H, C
2–C) δ 2.4 (s, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) δ 1.5 (s, 6H, 2 × C
3) δ 1.5 (s, 6H, 2 × C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); IR (cm−1) ν: 3292.03, 2992.75, 2931.93, 2858.50, 2114.38, 1067.29, 1025.50.
3); IR (cm−1) ν: 3292.03, 2992.75, 2931.93, 2858.50, 2114.38, 1067.29, 1025.50.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After around 30 min, 2,2,8-trimethyl-5-((prop-2-yn-1-yloxy)methyl)-4H-[1,3]dioxino[4,5-c]pyridine (3) (1 mmol) in absolute t-BuOH (1 mL) was added to the solution in the presence of CuSO4·5H2O (10 mol%) and freshly prepared ascorbic acid solution (25 mol%), at 50 °C and stirred at 70 °C for 12 h. After completion of the reaction (checked by using TLC), the reaction mixture was passed through a slurry of Celite in ethyl acetate. The filtrate was concentrated under reduced pressure. The organic residue was extracted with EtOAc (20 mL × 3). The organic phase was washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated to dryness under reduced pressure to afford compounds 4(a–q). The compounds were finally washed with hexane.
1). After around 30 min, 2,2,8-trimethyl-5-((prop-2-yn-1-yloxy)methyl)-4H-[1,3]dioxino[4,5-c]pyridine (3) (1 mmol) in absolute t-BuOH (1 mL) was added to the solution in the presence of CuSO4·5H2O (10 mol%) and freshly prepared ascorbic acid solution (25 mol%), at 50 °C and stirred at 70 °C for 12 h. After completion of the reaction (checked by using TLC), the reaction mixture was passed through a slurry of Celite in ethyl acetate. The filtrate was concentrated under reduced pressure. The organic residue was extracted with EtOAc (20 mL × 3). The organic phase was washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated to dryness under reduced pressure to afford compounds 4(a–q). The compounds were finally washed with hexane.4.1.5.1 5-(((1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5a). Brown oil; yield 95%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.75 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.75 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.39 (s, 1H, C
) δ 7.39 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.36–7.31 (m, 3H, Ar–
–N) δ 7.36–7.31 (m, 3H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.21 (dd, J = 7.2, 2.2, 2H, Ar–
) δ 7.21 (dd, J = 7.2, 2.2, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.45 (s, 2H, N–C
) δ 5.45 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.95 (s, 2H, C
2) δ 4.95 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.43 (s, 2H, C
2–O) δ 4.43 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.43 (s, 2H, C
2–OH) δ 4.43 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.40 (s, 3H, N–C–C
2–O) δ 2.40 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 152.05, 148.52, 144.80, 139.90, 134.26, 131.57, 129.28, 128.21, 127.54, 122.73, 68.25, 62.28, 59.77, 54.38, 18.67; IR (cm−1) ν: 3141.08, 2926.77, 2863.95, 2363.77, 2246.90, 1615.93, 1225.02, 1213.27; HRMS (m/z): calculated for C18H20N4O3 [M + H]+ 341.1691; found, 341.1611; HPLC purity-99%; tR = 9.86 min.
3); 13C NMR (125 MHz, chloroform-d) δ 152.05, 148.52, 144.80, 139.90, 134.26, 131.57, 129.28, 128.21, 127.54, 122.73, 68.25, 62.28, 59.77, 54.38, 18.67; IR (cm−1) ν: 3141.08, 2926.77, 2863.95, 2363.77, 2246.90, 1615.93, 1225.02, 1213.27; HRMS (m/z): calculated for C18H20N4O3 [M + H]+ 341.1691; found, 341.1611; HPLC purity-99%; tR = 9.86 min.
4.1.5.2 4-(Hydroxymethyl)-2-methyl-5-(((1-(4-methylbenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5b). Yellow sticky solid; yield 88%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.35 (s, 1H, C
) δ 7.35 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ = 7.14 (q, J = 8.1, 4H, Ar–
–N) δ = 7.14 (q, J = 8.1, 4H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.42 (s, 2H, N–C
) δ 5.42 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.97 (s, 2H, C
2) δ 4.97 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.52 (s, 2H, C
2–OH) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.42 (s, 2H, C
2–O) δ 4.42 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3HN–C–C
2–O) δ 2.45 (s, 3HN–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), δ 2.33 (s, 3H, Ar–C
3), δ 2.33 (s, 3H, Ar–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 144.76, 140.56, 139.15, 134.72, 131.53, 131.29, 130.05, 129.94, 128.28, 122.42, 68.35, 61.47, 59.67, 54.22, 21.23, 18.85. IR (cm−1) ν: 3145.19, 2930.77, 2869.75, 2367.37, 2248.92, 1620.39, 1221.03, 1210.28; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1772; HPLC purity-97%; tR = 11.20 min.
3); 13C NMR (125 MHz, chloroform-d) δ 144.76, 140.56, 139.15, 134.72, 131.53, 131.29, 130.05, 129.94, 128.28, 122.42, 68.35, 61.47, 59.67, 54.22, 21.23, 18.85. IR (cm−1) ν: 3145.19, 2930.77, 2869.75, 2367.37, 2248.92, 1620.39, 1221.03, 1210.28; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1772; HPLC purity-97%; tR = 11.20 min.
4.1.5.3 4-(Hydroxymethyl)-2-methyl-5-(((1-(3-methylbenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5c). Yellow oil; yield 87%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.37 (s, 1H, C
) δ 7.37 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.24–7.21 (m, 1H, Ar–
–N) δ 7.24–7.21 (m, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ = 7.16 (d, J = 7.6 Hz, 1H, Ar–
) δ = 7.16 (d, J = 7.6 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.05–7.02 (m, 2H, Ar–
) δ 7.05–7.02 (m, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.42 (s, 2H, N–C
) δ 5.42 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.97 (s, 2H, C
2) δ 4.97 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.52 (s, 2H, C
2–OH) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.43 (s, 2H, C
2–O) δ 4.43 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3H, N–C–C
2–O) δ 2.45 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), δ 2.32 (s, 3H, Ar–C
3), δ 2.32 (s, 3H, Ar–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 140.38, 139.18, 134.11, 131.64, 129.78, 129.16, 128.97, 127.45, 125.67, 125.31, 122.58, 68.34, 61.68, 59.60, 54.43, 21.39, 18.75. IR (cm−1) ν: 3146.20, 2931.47, 2868.75, 2369.58, 2249.22, 1622.19, 1223.10, 1211.32; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1768; HPLC purity-96%; tR = 10.30 min.
3); 13C NMR (125 MHz, chloroform-d) δ 140.38, 139.18, 134.11, 131.64, 129.78, 129.16, 128.97, 127.45, 125.67, 125.31, 122.58, 68.34, 61.68, 59.60, 54.43, 21.39, 18.75. IR (cm−1) ν: 3146.20, 2931.47, 2868.75, 2369.58, 2249.22, 1622.19, 1223.10, 1211.32; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1768; HPLC purity-96%; tR = 10.30 min.
4.1.5.4 4-(Hydroxymethyl)-2-methyl-5-(((1-(2-methylbenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5d). Dark-green semisolid; yield 93%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.29 (s, 1H, C
) δ 7.29 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ = 7.30–7.25 (m, 1H, Ar–
–N) δ = 7.30–7.25 (m, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.20 (dd, J = 7.2, 3.6 Hz, 2H, Ar–
), δ 7.20 (dd, J = 7.2, 3.6 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.13 (d, J = 8.0, 1H, Ar–
), δ 7.13 (d, J = 8.0, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.48 (s, 2H, N–C
) δ 5.48 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.97 (s, 2H, C
2) δ 4.97 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.52 (s, 2H, C
2–OH) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.42 (s, 2H, C
2–O) δ 4.42 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3H, N–C–C
2–O) δ 2.45 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), δ 2.24 (s, 3H, Ar–C
3), δ 2.24 (s, 3H, Ar–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 148.80, 143.86, 137.03, 132.11, 131.21, 129.61, 129.44, 126.83, 68.39, 61.62, 59.57, 52.56, 19.04; IR (cm−1) ν: 3147.20, 2930.70, 2870.59, 2372.85, 2247.31, 1620.20, 1221.70, 1215.36; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1774; HPLC purity-95%; tR = 10.92 min.
3); 13C NMR (125 MHz, chloroform-d) δ 148.80, 143.86, 137.03, 132.11, 131.21, 129.61, 129.44, 126.83, 68.39, 61.62, 59.57, 52.56, 19.04; IR (cm−1) ν: 3147.20, 2930.70, 2870.59, 2372.85, 2247.31, 1620.20, 1221.70, 1215.36; HRMS (m/z): calculated for C19H23N4O3 [M + H]+ 355.1770; found, 355.1774; HPLC purity-95%; tR = 10.92 min.
4.1.5.5 4-(Hydroxymethyl)-5-(((1-(3-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-2-methylpyridin-3-ol (5e). Yellow sticky solid; yield 90%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.78 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.78 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.40 (s, 1H, C
) δ 7.40 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.27 (d, J = 7.6 Hz, 1H, Ar–
–N) δ 7.27 (d, J = 7.6 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 6.86 (dd, J = 8.3, 2.5 Hz, 1H, Ar–
) δ 6.86 (dd, J = 8.3, 2.5 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 6.80 (d, J = 7.5 Hz, 1H, Ar–
) δ 6.80 (d, J = 7.5 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 6.74 (d, J = 1.6 Hz, 1H, Ar–
) δ 6.74 (d, J = 1.6 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.42 (s, 2H, N–C
) δ 5.42 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.96 (s, 2H, C
2) δ 4.96 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.46 (s, 2H, C
2–OH) δ 4.46 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.43 (s, 2H, C
2–O) δ 4.43 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 3.75 (s, 3H, Ar–O–C
2–O) δ 3.75 (s, 3H, Ar–O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), δ 2.42 (s, 3H, N–C–C
3), δ 2.42 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 156.30, 149.66, 144.88, 140.99, 134.97, 127.76, 124.99, 118.81, 118.55, 72.91, 68.02, 64.64, 60.09, 58.73, 23.86; IR (cm−1) ν: 3248.36, 2932.56, 2873.62, 2375.56, 2248.32, 1620.20, 1231.80, 1218.36; HRMS (m/z): calculated for C19H23N4O4 [M + H]+ 371.1719; found, 371.1719; HPLC purity-96%; tR = 10.24 min.
3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 156.30, 149.66, 144.88, 140.99, 134.97, 127.76, 124.99, 118.81, 118.55, 72.91, 68.02, 64.64, 60.09, 58.73, 23.86; IR (cm−1) ν: 3248.36, 2932.56, 2873.62, 2375.56, 2248.32, 1620.20, 1231.80, 1218.36; HRMS (m/z): calculated for C19H23N4O4 [M + H]+ 371.1719; found, 371.1719; HPLC purity-96%; tR = 10.24 min.
4.1.5.6 5-(((1-(3,5-Dimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5f). Yellow oil; yield 85%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.83 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.41 (s, 1H, C
) δ 7.41 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.25 (s, 1H, Ar–
–N) δ 7.25 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 6.40 (s, 1H, Ar–
) δ 6.40 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 6.36 (s, 1H, Ar–
), δ 6.36 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 5.39 (s, 2H, N–C
), δ 5.39 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.96 (s, 2H, C
2) δ 4.96 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.50 (s, 2H, C
2–OH) δ 4.50 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.44 (s, 2H, C
2–O) δ 4.44 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 3.74 (s, 6H, Ar–O–C
2–O) δ 3.74 (s, 6H, Ar–O–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), δ 2.44 (s, 3H, N–C–C
3), δ 2.44 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 161.43, 136.37, 127.44, 122.72, 106.32, 100.48, 68.30, 61.88, 59.74, 55.54, 54.41, 18.79.; IR (cm−1) ν: 3245.63, 2893.60, 2892.36, 2376.78, 2369.32, 1623.20, 1241.81, 1278.23; HRMS (m/z): calculated for C20H25N4O5 [M + H]+ 401.1825; found, 401.1834; HPLC purity-97%; tR = 10.72 min.
3); 13C 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 161.43, 136.37, 127.44, 122.72, 106.32, 100.48, 68.30, 61.88, 59.74, 55.54, 54.41, 18.79.; IR (cm−1) ν: 3245.63, 2893.60, 2892.36, 2376.78, 2369.32, 1623.20, 1241.81, 1278.23; HRMS (m/z): calculated for C20H25N4O5 [M + H]+ 401.1825; found, 401.1834; HPLC purity-97%; tR = 10.72 min.
4.1.5.7 5-(((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5g). Brown oil; yield 93%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.38 (s, 1H, C
) δ 7.38 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.24–7.20 (m, 2H, Ar–
–N) δ 7.24–7.20 (m, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.03 (t, J = 8.6 Hz, 2H, Ar–
), δ 7.03 (t, J = 8.6 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.44 (s, 2H, N–C
) δ 5.44 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.51 (s, 2H, C
2–OH) δ 4.51 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.44 (s, 2H, C
2–O) δ 4.44 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.44 (s, 3H, N–C–C
2–O) δ 2.44 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 148.67, 145.34, 140.30, 131.58, 130.16, 130.09, 122.49, 116.41, 116.24, 68.38, 61.78, 59.64, 53.64, 18.75; 19F NMR (470 MHz, chloroform-d) δ −112.10 to −112.17 (m); IR (cm−1) ν: 3247.63, 2896.70, 2912.86, 2378.87, 2367.85, 1613.10, 1245.81, 1218.23; HRMS (m/z): calculated for C18H20N4O3F [M + H]+ 359.1519; found, 359.1514; HPLC purity-99.8%; tR = 10.32 min.
3); 13C NMR (125 MHz, chloroform-d) δ 148.67, 145.34, 140.30, 131.58, 130.16, 130.09, 122.49, 116.41, 116.24, 68.38, 61.78, 59.64, 53.64, 18.75; 19F NMR (470 MHz, chloroform-d) δ −112.10 to −112.17 (m); IR (cm−1) ν: 3247.63, 2896.70, 2912.86, 2378.87, 2367.85, 1613.10, 1245.81, 1218.23; HRMS (m/z): calculated for C18H20N4O3F [M + H]+ 359.1519; found, 359.1514; HPLC purity-99.8%; tR = 10.32 min.
4.1.5.8 5-(((1-(3-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5h). Yellow oil; yield 86%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.81 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.81 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.42 (s, 1H, C
) δ 7.42 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.33 (td, J = 8.0, 5.9 Hz, 1H, Ar–
–N) δ 7.33 (td, J = 8.0, 5.9 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.06–6.99 (m, 2H, Ar–
), δ 7.06–6.99 (m, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 6.92 (d, J = 9.1 Hz, 1H, Ar–
) δ 6.92 (d, J = 9.1 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.47 (s, 2H, N–C
) δ 5.47 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.51 (s, 2H, C
2–OH) δ 4.51 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.45 (s, 2H, C
2–O) δ 4.45 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.44 (s, 3H, N–C–C
2–O) δ 2.44 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 151.98, 148.68, 145.06, 140.25, 131.55, 131.00, 130.94, 127.45, 123.65, 122.69, 116.15, 115.99, 115.24, 115.07, 68.37, 61.86, 59.69, 53.72, 18.73; 19F NMR (470 MHz, chloroform-d) δ −111.20 (ddd, J = 9.0, 6.3, 2.2 Hz); IR (cm−1) ν: 3245.63, 2893.70, 2911.86, 2375.77, 2377.65, 1619.20, 1286.81, 1220.23; HRMS (m/z): calculated for C18H20N4O3F [M + H]+ 359.1519; found, 359.1519; HPLC purity-98%; tR = 10.30 min.
3); 13C NMR (125 MHz, chloroform-d) δ 151.98, 148.68, 145.06, 140.25, 131.55, 131.00, 130.94, 127.45, 123.65, 122.69, 116.15, 115.99, 115.24, 115.07, 68.37, 61.86, 59.69, 53.72, 18.73; 19F NMR (470 MHz, chloroform-d) δ −111.20 (ddd, J = 9.0, 6.3, 2.2 Hz); IR (cm−1) ν: 3245.63, 2893.70, 2911.86, 2375.77, 2377.65, 1619.20, 1286.81, 1220.23; HRMS (m/z): calculated for C18H20N4O3F [M + H]+ 359.1519; found, 359.1519; HPLC purity-98%; tR = 10.30 min.
4.1.5.9 5-(((1-(3,5-Difluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5i). Yellow sticky solid; yield 96%; Rf = 0.3 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d + DMSO-d6) δ 7.87 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d + DMSO-d6) δ 7.87 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.48 (s, 1H, C
) δ 7.48 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 6.85–6.79 (m, 3H, Ar–
–N) δ 6.85–6.79 (m, 3H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 5.56 (s, 2H, N–C
), δ 5.56 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.91 (s, 2H, C
2) δ 4.91 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.60 (s, 2H, C
2–OH) δ 4.60 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.52 (s, 2H, C
2–O) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.42 (s, 3H, N–C–C
2–O) δ 2.42 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 156.20, 149.88, 144.87, 143.80, 135.68, 132.50, 128.31, 115.92, 115.71, 108.78, 72.95, 68.04, 64.48, 57.54, 23.92; 19F NMR (470 MHz, chloroform-d) δ −103.42 to −103.51 (m); IR (cm−1) ν: 3240.35, 2885.91, 2900.62, 2378.85, 2378.63, 1622.31, 1278.78, 1223.73; HRMS (m/z): calculated for C18H19N4O3F2 [M + H]+ 377.1425; found, 377.1422; HPLC purity-100%; tR = 10.88 min.
3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 156.20, 149.88, 144.87, 143.80, 135.68, 132.50, 128.31, 115.92, 115.71, 108.78, 72.95, 68.04, 64.48, 57.54, 23.92; 19F NMR (470 MHz, chloroform-d) δ −103.42 to −103.51 (m); IR (cm−1) ν: 3240.35, 2885.91, 2900.62, 2378.85, 2378.63, 1622.31, 1278.78, 1223.73; HRMS (m/z): calculated for C18H19N4O3F2 [M + H]+ 377.1425; found, 377.1422; HPLC purity-100%; tR = 10.88 min.
4.1.5.10 5-(((1-(4-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5j). Yellow sticky solid; yield 85%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.80 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.80 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.40 (s, 1H, C
) δ 7.40 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.32 (d, J = 8.4 Hz, 2H, Ar–
–N) δ 7.32 (d, J = 8.4 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.16 (d, J = 8.3 Hz, 2H, Ar–
), δ 7.16 (d, J = 8.3 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.43 (s, 2H, N–C
) δ 5.43 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.97 (s, 2H, C
2) δ 4.97 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.48 (s, 2H, C
2–OH) δ 4.48 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.44 (s, 2H, C
2–O) δ 4.44 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.43 (s, 3H, N–C–C
2–O) δ 2.43 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 148.53, 145.03, 139.96, 135.10, 132.76, 131.63, 129.53, 129.50, 127.57, 122.62, 68.32, 62.04, 59.72, 53.62, 29.78, 18.65; IR (cm−1) ν: 3224.69, 2895.81, 2910.25, 2376.68, 2379.36, 1627.91, 1278.70, 1210.35; HRMS (m/z): calculated for C18H20N4O3Cl [M + H]+ 375.1224; found, 375.1222; HPLC purity-97%; tR = 11.56 min.
3); 13C NMR (125 MHz, chloroform-d) δ 148.53, 145.03, 139.96, 135.10, 132.76, 131.63, 129.53, 129.50, 127.57, 122.62, 68.32, 62.04, 59.72, 53.62, 29.78, 18.65; IR (cm−1) ν: 3224.69, 2895.81, 2910.25, 2376.68, 2379.36, 1627.91, 1278.70, 1210.35; HRMS (m/z): calculated for C18H20N4O3Cl [M + H]+ 375.1224; found, 375.1222; HPLC purity-97%; tR = 11.56 min.
4.1.5.11 5-(((1-(3-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5k). Yellow oil; yield 88%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.84 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.84 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.42 (s, 1H, C
) δ 7.42 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.34–7.27 (m, 2H, Ar–
–N) δ 7.34–7.27 (m, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.23 (s, 1H, Ar–
), δ 7.23 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.10 (d, J = 7.2 Hz, 1H) δ 5.45 (s, 2H, N–C
) δ 7.10 (d, J = 7.2 Hz, 1H) δ 5.45 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.99 (s, 2H, C
2) δ 4.99 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.54 (s, 2H, C
2–OH) δ 4.54 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.45 (s, 2H, C
2–O) δ 4.45 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3H, N–C–C
2–O) δ 2.45 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 151.92, 149.51, 148.78, 145.10, 130.60, 129.26, 128.25, 126.24, 122.65, 68.43, 61.68, 59.66, 53.67, 18.81; IR (cm−1) ν: 3220.69, 2898.81, 2914.38, 2369.82, 2381.41, 1628.85, 1280.01, 1210.56; HRMS (m/z): calculated for C18H20N4O3Cl [M + H]+ 375.1229; found, 375.1225; HPLC purity-97%; tR = 10.32 min.
3); 13C NMR (125 MHz, chloroform-d) δ 151.92, 149.51, 148.78, 145.10, 130.60, 129.26, 128.25, 126.24, 122.65, 68.43, 61.68, 59.66, 53.67, 18.81; IR (cm−1) ν: 3220.69, 2898.81, 2914.38, 2369.82, 2381.41, 1628.85, 1280.01, 1210.56; HRMS (m/z): calculated for C18H20N4O3Cl [M + H]+ 375.1229; found, 375.1225; HPLC purity-97%; tR = 10.32 min.
4.1.5.12 5-(((1-(3,4-Dichlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5l). Yellow oil; yield 92%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d + DMSO-d6) δ 7.75 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d + DMSO-d6) δ 7.75 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.64 (s, 1H, C
) δ 7.64 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.24–7.20 (m, 2H, Ar–
–N) δ 7.24–7.20 (m, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), δ 7.06 (s, 1H, Ar–
), δ 7.06 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.40 (s, 2H, N–C
) δ 5.40 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.78 (s, 2H, C
2) δ 4.78 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.48 (s, 2H, C
2–OH) δ 4.48 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.38 (s, 2H, C
2–O) δ 4.38 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.30 (s, 3H, N–C–C
2–O) δ 2.30 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 135.45, 131.07, 130.10, 127.62, 123.37, 68.19, 64.43, 59.76, 52.57, 19.27; IR (cm−1) ν: 3225.72, 2890.23, 2926.83, 2370.82, 2380.62, 1629.85, 1282.01, 1217.61; HRMS (m/z): calculated for C18H19N4O3Cl2 [M + H]+ 410.0912; found, 410.0865; HPLC purity-98%; tR = 10.32 min.
3); 13C NMR (125 MHz, chloroform-d and DMSO-d6) δ 135.45, 131.07, 130.10, 127.62, 123.37, 68.19, 64.43, 59.76, 52.57, 19.27; IR (cm−1) ν: 3225.72, 2890.23, 2926.83, 2370.82, 2380.62, 1629.85, 1282.01, 1217.61; HRMS (m/z): calculated for C18H19N4O3Cl2 [M + H]+ 410.0912; found, 410.0865; HPLC purity-98%; tR = 10.32 min.
4.1.5.13 5-(((1-(4-Bromobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5m). Yellow sticky solid; yield 91%; Rf = 0.6 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.84 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.84 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.49 (d, J = 8.3 Hz, 2H, Ar–
) δ 7.49 (d, J = 8.3 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.48 (s, 1H, C
) δ 7.48 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.11 (d, J = 8.3, 2H, Ar–
–N) δ 7.11 (d, J = 8.3, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.43 (s, 2H, N–C
) δ 5.43 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.53 (s, 2H, C
2–OH) δ 4.53 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.44 (s, 2H, C
2–O) δ 4.44 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3H, N–C–C
2–O) δ 2.45 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) 13C NMR (125 MHz, chloroform-d) δ 148.75, 133.25, 132.48, 129.80, 123.24, 122.56, 68.42, 61.70, 59.64, 53.69, 29.78; IR (cm−1) ν: 3226.24, 2892.83, 2924.81, 2374.22, 2386.92, 1685.23, 1280.81, 1216.56; HRMS (m/z): calculated for C18H20N4O3Br [M + H]+ 419.0719; found, 419.0715; HPLC purity-99%; tR = 11.91 min.
3) 13C NMR (125 MHz, chloroform-d) δ 148.75, 133.25, 132.48, 129.80, 123.24, 122.56, 68.42, 61.70, 59.64, 53.69, 29.78; IR (cm−1) ν: 3226.24, 2892.83, 2924.81, 2374.22, 2386.92, 1685.23, 1280.81, 1216.56; HRMS (m/z): calculated for C18H20N4O3Br [M + H]+ 419.0719; found, 419.0715; HPLC purity-99%; tR = 11.91 min.
4.1.5.14 5-(((1-(3-Bromobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)-4-(hydroxymethyl)-2-methylpyridin-3-ol (5n). Yellow oil; yield 91%; Rf = 0.6 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.85 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.85 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.48 (s, 1H, C
) δ 7.48 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.41 (d, J = 8.3 Hz, 2H, Ar–
–N) δ 7.41 (d, J = 8.3 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.23 (s, 1H, Ar–
) δ 7.23 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.15 (d, J = 7.7, 1H, Ar–
) δ 7.15 (d, J = 7.7, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.44 (s, 2H, N–C
) δ 5.44 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.53 (s, 2H, C
2–OH) δ 4.53 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.45 (s, 2H, C
2–O) δ 4.45 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.45 (s, 3H, N–C–C
2–O) δ 2.45 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 132.20, 131.15, 130.85, 126.72, 123.30, 117.51, 68.44, 61.74, 59.67, 53.60, 36.88 IR (cm−1) ν: 3225.20, 2890.63, 2923.72, 2373.15, 2388.85, 1685.57, 1282.74, 1210.78; HRMS (m/z): calculated for C18H20N4O3Br [M + H]+ 419.0719; found, 419.0715; HPLC purity-95%; tR = 11.79 min.
3); 13C NMR (125 MHz, chloroform-d) δ 132.20, 131.15, 130.85, 126.72, 123.30, 117.51, 68.44, 61.74, 59.67, 53.60, 36.88 IR (cm−1) ν: 3225.20, 2890.63, 2923.72, 2373.15, 2388.85, 1685.57, 1282.74, 1210.78; HRMS (m/z): calculated for C18H20N4O3Br [M + H]+ 419.0719; found, 419.0715; HPLC purity-95%; tR = 11.79 min.
4.1.5.15 4-(Hydroxymethyl)-2-methyl-5-(((1-(4-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5o). Yellow sticky solid; yield 95%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.80 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.80 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.62 (d, J = 8.2 Hz, 2H, Ar–
) δ 7.62 (d, J = 8.2 Hz, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.43 (s, 1H, C
) δ 7.43 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) 7.33 (d, J = 8.1, 2H, Ar–
–N) 7.33 (d, J = 8.1, 2H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.53 (s, 2H, N–C
) δ 5.53 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.49 (s, 2H, C
2–OH) δ 4.49 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.45 (s, 2H, C
2–O) δ 4.45 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.43 (s, 3H, N–C–C
2–O) δ 2.43 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 151.97, 148.67, 145.22, 140.19, 138.21, 131.49, 128.35, 127.46, 126.26, 122.75, 68.40, 61.94, 59.72, 53.67, 18.74; 19F NMR (470 MHz, chloroform-d) δ −62.71 (s); IR (cm−1) ν: 3115.52, 2895.96, 2925.63, 2375.38, 2390.58, 1182.06, 1683.57, 1281.54, 1213.61; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1489; HPLC purity-97%; tR = 12.57 min.
3); 13C NMR (125 MHz, chloroform-d) δ 151.97, 148.67, 145.22, 140.19, 138.21, 131.49, 128.35, 127.46, 126.26, 122.75, 68.40, 61.94, 59.72, 53.67, 18.74; 19F NMR (470 MHz, chloroform-d) δ −62.71 (s); IR (cm−1) ν: 3115.52, 2895.96, 2925.63, 2375.38, 2390.58, 1182.06, 1683.57, 1281.54, 1213.61; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1489; HPLC purity-97%; tR = 12.57 min.
4.1.5.16 4-(Hydroxymethyl)-2-methyl-5-(((1-(3-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5p). Yellow oil; yield 87%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.62 (s, 1H, C
) δ 7.62 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.51 (d, J = 4.4 Hz, 1H, Ar–
–N) δ 7.51 (d, J = 4.4 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.49 (d, J = 7.8 Hz, 1H, Ar–
) δ 7.49 (d, J = 7.8 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.44 (s, 1H, Ar–
) δ 7.44 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.41 (d, J = 7.7 Hz, 1H, Ar–
) δ 7.41 (d, J = 7.7 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.54 (s, 2H, N–C
) δ 5.54 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.52 (s, 2H, C
2–OH) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.46 (s, 2H, C
2–O) δ 4.46 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.44 (s, 3H, N–C–C
2–O) δ 2.44 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) 13C NMR (125 MHz, chloroform-d) δ 140.23, 140.11, 135.32, 131.44, 129.95, 125.88, 124.84, 122.68, 68.40, 59.69, 53.74, 29.78; 19F NMR (470 MHz, chloroform-d) δ −62.64 (s); IR (cm−1) ν: 3116.27, 2896.56, 2923.18, 2374.38, 2391.60, 1180.28, 1682.66, 1280.45, 1216.68; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1492; HPLC purity-96%; tR = 8.70 min.
3) 13C NMR (125 MHz, chloroform-d) δ 140.23, 140.11, 135.32, 131.44, 129.95, 125.88, 124.84, 122.68, 68.40, 59.69, 53.74, 29.78; 19F NMR (470 MHz, chloroform-d) δ −62.64 (s); IR (cm−1) ν: 3116.27, 2896.56, 2923.18, 2374.38, 2391.60, 1180.28, 1682.66, 1280.45, 1216.68; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1492; HPLC purity-96%; tR = 8.70 min.
4.1.5.17 4-(Hydroxymethyl)-2-methyl-5-(((1-(2-(trifluoromethyl)benzyl)-1H-1,2,3-triazol-4-yl)methoxy)methyl)pyridin-3-ol (5q). Brown oil; yield 89%; Rf = 0.4 (DCM/methanol 19
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N
1); 1H NMR (500 MHz, chloroform-d) δ 7.82 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.62 (s, 1H, C
) δ 7.62 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –N) δ 7.51 (d, J = 4.4 Hz, 1H, Ar–
–N) δ 7.51 (d, J = 4.4 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.49 (d, J = 7.8 Hz, 1H, Ar–
) δ 7.49 (d, J = 7.8 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.44 (s, 1H, Ar–
) δ 7.44 (s, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 7.40 (d, J = 7.7 Hz, 1H, Ar–
) δ 7.40 (d, J = 7.7 Hz, 1H, Ar–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) δ 5.54 (s, 2H, N–C
) δ 5.54 (s, 2H, N–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) δ 4.98 (s, 2H, C
2) δ 4.98 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) δ 4.52 (s, 2H, C
2–OH) δ 4.52 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 4.46 (s, 2H, C
2–O) δ 4.46 (s, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O) δ 2.44 (s, 3H, N–C–C
2–O) δ 2.44 (s, 3H, N–C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3); 13C NMR (125 MHz, chloroform-d) δ 152.85, 132.87, 130.47, 129.06, 126.41, 123.04, 68.43, 61.65, 59.65, 50.39, 19.04; 19F NMR (470 MHz, chloroform-d) δ −58.88 (s); IR (cm−1) ν: 3117.83, 2897.75, 2927.20, 2378.42, 2390.56, 1185.75, 1683.20, 1283.30, 1215.78; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1490; HPLC purity-98%; tR = 12.09 min.
3); 13C NMR (125 MHz, chloroform-d) δ 152.85, 132.87, 130.47, 129.06, 126.41, 123.04, 68.43, 61.65, 59.65, 50.39, 19.04; 19F NMR (470 MHz, chloroform-d) δ −58.88 (s); IR (cm−1) ν: 3117.83, 2897.75, 2927.20, 2378.42, 2390.56, 1185.75, 1683.20, 1283.30, 1215.78; HRMS (m/z): calculated for C19H20N4O3F3 [M + H]+ 409.1488; found, 409.1490; HPLC purity-98%; tR = 12.09 min.
4.2 Biological activity
4.3 Metal chelation study
Metal chelation studies of the tested compounds were done using UV spectrophotometer (Agilent Technologies 500) within the wavelength range of 200 to 600 nm at 37 °C. Solutions of FeCl3, CuCl2, ZnSO4·H2O, AlCl3·6H2O, KCl, NaCl, CaSO4, Mg(OH)2·6H2O and compounds to be tested were prepared, such that the resulting solution comprised 100 μM each of ligand and metal and incubated at 37 °C for 30 min. For taking the spectra of the compounds alone, metal solution was replaced with Milli Q water.For affinity study of 5i towards Fe, all metals Fe3+, Zn2+, Cu2+, Ca2+, Mg2+, Na+ and K+ were mixed, such that the reaction mixture contained equal concentration each including the ligand 5i as 100 μM.
For stoichiometric studies, 100 μM solution of 5i was taken in the cuvette and to it 20 μL aliquots of 1 mM FeCl3 was added till the final concentration reached of metal reached 100 μM. The concentration of FeCl3 ranged from 10–100 μM and the titration was done in 37 °C.
4.4 In silico study
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Tiyas Pal and Saipriyanka Bhimaneni are thankful to Department of Pharmaceutical, Ministry of Chemicals and Fertilizers for providing financial assistance. Spectral study was carried out at Central Instrumental Facility NIPER-R. NIPER-R/Communication/123.References
- P. Christina, World Alzheimer's Report 2018, Alzheimer's Dis. Int. world Alzheimer Rep., 2018, pp. 1–48 Search PubMed.
- A. Cavalli, M. L. Bolognesi, A. Minarini, M. Rosini, V. Tumiatti, M. Recanatini and C. Melchiorre, Multi-Target-Directed Ligands to Combat Neurodegenerative Diseases, J. Med. Chem., 2008, 51(7), 2326 CrossRef CAS.
- R. R. Ramsay, M. R. Popovic-Nikolic, K. Nikolic, E. Uliassi and M. L. Bolognesi, A Perspective on Multi-Target Drug Discovery and Design for Complex Diseases, Clin. Transl. Med., 2018, 7(3), 1–14 Search PubMed.
- P. Mecocci and M. C. Polidori, Antioxidant Clinical Trials in Mild Cognitive Impairment and Alzheimer's Disease, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822(5), 631–638 CrossRef CAS PubMed.
- M. A. Silva, A. S. Kiametis and W. Treptow, Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature, J. Chem. Inf. Model., 2020 DOI:10.1021/acs.jcim.9b01073.
- H. Tachallait, A. Bouyahya, A. Talha, Y. Bakri, N. Dakka, L. Demange, R. Benhida and K. Bougrin, Concise Synthesis and Antibacterial Evaluation of Novel 3-(1,4-Disubstituted-1,2,3-Triazolyl)Uridine Nucleosides, Arch. Pharm., 2018, 351(11), 1–11 CrossRef PubMed.
- A. Aziz Ali, D. Gogoi, A. K. Chaliha, A. K. Buragohain, P. Trivedi, P. J. Saikia, P. S. Gehlot, A. Kumar, V. Chaturvedi and D. Sarma, Synthesis and Biological Evaluation of Novel 1,2,3-Triazole Derivatives as Anti-Tubercular Agents, Bioorg. Med. Chem. Lett., 2017, 27(16), 3698–3703 CrossRef CAS PubMed.
- M. Irfan, B. Aneja, U. Yadava, S. I. Khan, N. Manzoor, C. G. Daniliuc and M. Abid, Synthesis, QSAR and Anticandidal Evaluation of 1,2,3-Triazoles Derived from Naturally Bioactive Scaffolds, Eur. J. Med. Chem., 2015, 93, 246–254 CrossRef CAS PubMed.
- A. Rastegari, H. Nadri, M. Mahdavi, A. Moradi, S. Sara, N. Edraki, F. Homayouni and B. Larijani, Bioorganic Chemistry Design, Synthesis and Anti-Alzheimer's Activity of Novel 1,2,3-Triazole-Chromenone Carboxamide Derivatives, Bioorg. Chem., 2019, 83, 391–401 CrossRef CAS PubMed.
- L. Jalili-Baleh, H. Nadri, H. Forootanfar, A. Samzadeh-Kermani, T. T. Küçükkılınç, B. Ayazgok, M. Rahimifard, M. Baeeri, M. Doostmohammadi, L. Firoozpour, S. N. A. Bukhari, M. Abdollahi, M. R. Ganjali, S. Emami, M. Khoobi and A. Foroumadi, Novel 3-Phenylcoumarin–Lipoic Acid Conjugates as Multi-Functional Agents for Potential Treatment of Alzheimer's Disease, Bioorg. Chem., 2018, 79, 223–234 CrossRef CAS PubMed.
- X. Yang, X. Qiang, Y. Li, L. Luo, R. Xu, Y. Zheng, Z. Cao, Z. Tan and Y. Deng, Pyridoxine-Resveratrol Hybrids Mannich Base Derivatives as Novel Dual Inhibitors of AChE and MAO-B with Antioxidant and Metal-Chelating Properties for the Treatment of Alzheimer's Disease, Bioorg. Chem., 2017, 71, 305–314 CrossRef CAS PubMed.
- M. Yazdani, N. Edraki, R. Badri, M. Khoshneviszadeh, A. Iraji and O. Firuzi, Multi-Target Inhibitors against Alzheimer Disease Derived from 3-Hydrazinyl 1,2,4-Triazine Scaffold Containing Pendant Phenoxy Methyl-1,2,3-Triazole: Design, Synthesis and Biological Evaluation, Bioorg. Chem., 2019, 84, 363–371 CrossRef CAS PubMed.
- Z. Najafi, M. Mahdavi, M. Saeedi, E. Karimpour-Razkenari, R. Asatouri, F. Vafadarnejad, F. H. Moghadam, M. Khanavi, M. Sharifzadeh and T. Akbarzadeh, Novel Tacrine-1,2,3-Triazole Hybrids: In vitro, In vivo Biological Evaluation and Docking Study of Cholinesterase Inhibitors, Eur. J. Med. Chem., 2017, 125, 1200–1212 CrossRef CAS PubMed.
- A. Kaur, S. S. Narang, A. Kaur, S. Mann, N. Priyadarshi, B. Goyal, N. K. Singhal and D. Goyal, Multifunctional Mono-Triazole Derivatives Inhibit Aβ42 Aggregation and Cu2+-Mediated Aβ42 Aggregation and Protect against Aβ42-Induced Cytotoxicity, Chem. Res. Toxicol., 2019, 32(9), 1824–1839 Search PubMed.
- M. R. Jones, E. L. Service, J. R. Thompson, M. C. P. Wang, I. J. Kimsey, A. S. Detoma, A. Ramamoorthy, M. H. Lim and T. Storr, Dual-Function Triazole-Pyridine Derivatives as Inhibitors of Metal-Induced Amyloid-β Aggregation, Metallomics, 2012, 4(9), 910–920 RSC.
- J. M. Zhuo and D. Praticò, Acceleration of Brain Amyloidosis in an Alzheimer's Disease Mouse Model by a Folate, Vitamin B6 and B12-Deficient Diet, Exp. Gerontol., 2010, 45(3), 195–201 CrossRef CAS PubMed.
- A. Hashim, L. Wanga, K. Junej, Y. Yeb, Y. Zhao and L. J. Ming, Vitamin B6s Inhibit Oxidative Stress Caused by Alzheimer's Disease-Related CuII-b-Amyloid Complexes-Cooperative Action of Phospho-Moiety, Bioorg. Med. Chem. Lett., 2011, 21(21), 6430–6432 CrossRef CAS PubMed.
- L. Drtinova, P. Dobes and M. Pohanka, Low Molecular Weight Precursor Applicable for Alzheimer Disease Drugs Synthesis (AChE and BChE Inhibition, BACE Inhibition, Antioxidant Properties and in Silico Modulation), J. Appl. Biomed., 2014, 12(4), 285–290 CrossRef.
- A. Campbell, The Potential Role of Aluminium in Alzheimer's Disease, Nephrol., Dial., Transplant., 2002, 17, 17–20 CrossRef CAS PubMed.
- P. F. Good, D. P. Perl, L. M. Bierer and J. Schmeidler, Selective Accumulation of Aluminum and Iron in the Neurofibrillary Tangles of Alzheimer's Disease: A Laser Microprobe (LAMMA) Study, Ann. Neurol., 1992, 31(3), 286–292 CrossRef CAS PubMed.
- H. Akrami, B. F. Mirjalili, M. Khoobi, H. Nadri, A. Moradi, A. Sakhteman, S. Emami, A. Foroumadi and A. Shafiee, Indolinone-Based Acetylcholinesterase Inhibitors: Synthesis, Biological Activity and Molecular Modeling, Eur. J. Med. Chem., 2014, 84, 375–381 CrossRef CAS PubMed.
- G. Johnson and S. Moore, The Peripheral Anionic Site of Acetylcholinesterase: Structure, Functions and Potential Role in Rational Drug Design, Curr. Pharm. Des., 2005, 12(2), 217–225 CrossRef PubMed.
- A. Daina and V. Zoete, A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules, ChemMedChem, 2016, 1117–1121 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules, Sci. Rep., 2017, 7, 1–13 CrossRef PubMed.
- M. N. Drwal, P. Banerjee, M. Dunkel, M. R. Wettig and R. Preissner, ProTox: A Web Server for the in Silico Prediction of Rodent Oral Toxicity, Nucleic Acids Res., 2014, 42, 3–8 CrossRef PubMed.
- N. L. Morozowich, A. L. Weikel, J. L. Nichol, C. Chen, L. S. Nair, C. T. Laurencin and H. R. Allcock, Polyphosphazenes Containing Vitamin Substituents: Synthesis, Characterization, and Hydrolytic Sensitivity, Macromolecules, 2011, 44(6), 1355–1364 CrossRef CAS.
- M. Pohanka, M. Hrabinova, K. Kuca and J. P. Simonato, Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman's Method, Int. J. Mol. Sci., 2011, 12(4), 2631–2640 CrossRef CAS PubMed.
- K. Pérez-Cruz, M. Moncada-Basualto, J. Morales-Valenzuela, G. Barriga-González, P. Navarrete-Encina, L. Núñez-Vergara, J. A. Squella and C. Olea-Azar, Synthesis and Antioxidant Study of New Polyphenolic Hybrid-Coumarins, Arabian J. Chem., 2018, 11(4), 525–537 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Spectra data. See DOI: 10.1039/d0ra04942e |
| This journal is © The Royal Society of Chemistry 2020 |