Themed collection Organic Synthesis

α-Borylalkyl radicals: their distinctive reactivity in modern organic synthesis
In this review, we emphasise the importance of the generation of α-boryl carbon-centred radicals and their utilisation in synthesis.

Chem. Commun., 2020,56, 13-25
https://doi.org/10.1039/C9CC08027A
Gold and hypervalent iodine(III): liaisons over a decade for electrophilic functional group transfer reactions
Building on mechanistic perspective, the review intends to demonstrate how the uniqueness of Au-catalysts has realized a myriad of electrophilic functional group transfer reactions with the use of hypervalent iodine(III) reagents over the last decade.
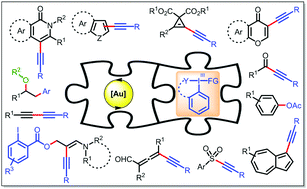
Chem. Commun., 2020,56, 2677-2690
https://doi.org/10.1039/D0CC00106F
Recent advances in metal-catalysed asymmetric sequential double hydrofunctionalization of alkynes
Recent advances in various metal-catalysed asymmetric sequential double hydrofunctionalizations of alkynes have been highlighted in this feature article.
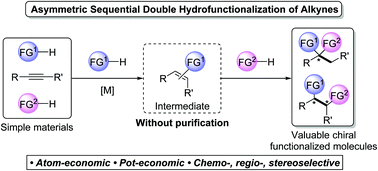
Chem. Commun., 2020,56, 2229-2239
https://doi.org/10.1039/D0CC00068J
Twenty-five years of bis-pentafluorophenyl borane: a versatile reagent for catalyst and materials synthesis
Highlights of the extensive chemistry and applications of bis-pentafluorophenyl borane (“Piers’ borane”) from the 25 years since its first appearance are featured.
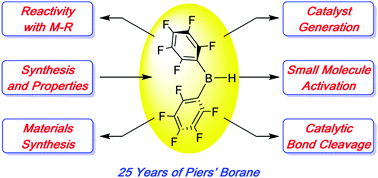
Chem. Commun., 2020,56, 841-853
https://doi.org/10.1039/C9CC08338C
Photoinduced deaminative strategies: Katritzky salts as alkyl radical precursors
Primary amines are one of the most predominant functional groups found in organic molecules. This review covers the most recent developments on photocatalytic deaminative strategies by using Katritzky Salts as alkyl radical reservoirs.
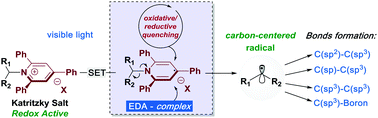
Chem. Commun., 2020,56, 503-514
https://doi.org/10.1039/C9CC08348K
Transition metal-catalyzed sp3 C–H activation and intramolecular C–N coupling to construct nitrogen heterocyclic scaffolds
Nitrogen heterocycles are of great medicinal importance, and the construction of nitrogen heterocyclic scaffolds has been one of the focuses in synthetic organic chemistry.
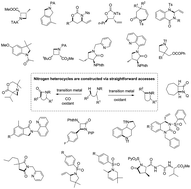
Chem. Commun., 2019,55, 13048-13065
https://doi.org/10.1039/C9CC06609H
Enantioselective synthesis of multi-nitrogen-containing heterocycles using azoalkenes as key intermediates
The recently developed annulation reactions using azoalkenes as key intermediates show their great ability to construct diverse types of multi-nitrogen-containing heterocycles. In this feature article, we critically analysed the strategic development and the efficient transformation of azoalkenes to chiral heterocycles and α-functionalized ketone derivatives since 2010.
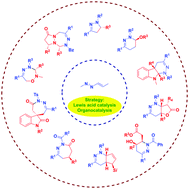
Chem. Commun., 2019,55, 6672-6684
https://doi.org/10.1039/C9CC02371B
Continuous flow chemistry: where are we now? Recent applications, challenges and limitations
A general outlook of the changing face of chemical synthesis is provided in this article through recent applications of continuous flow processing in both industry and academia.
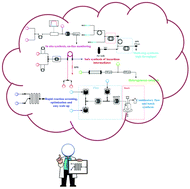
Chem. Commun., 2018,54, 13894-13928
https://doi.org/10.1039/C8CC07427E
Copper-catalysed ortho-selective C–H bond functionalization of phenols and naphthols with α-aryl-α-diazoesters
An unprecedented CuCl2-catalysed chemo- and ortho-selective C–H bond functionalization of phenols and naphthols with diazoesters has been developed.
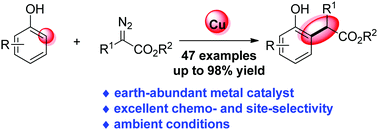
Chem. Commun., 2020,56, 9485-9488
https://doi.org/10.1039/D0CC04495D
Combination of organocatalytic oxidation of alcohols and organolithium chemistry (RLi) in aqueous media, at room temperature and under aerobic conditions
Organocatalysis and highly-polar s-block organometallic chemistry (RLi) work together in water, under air and at room temperature for the selective and ultrafast synthesis of tertiary alcohols.
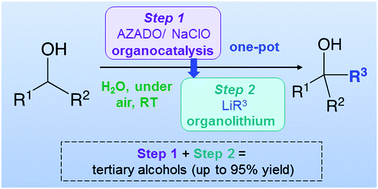
Chem. Commun., 2020,56, 8932-8935
https://doi.org/10.1039/D0CC03768K
Regioselective biocatalytic self-sufficient Tishchenko-type reaction via formal intramolecular hydride transfer
Alcohol dehydrogenases catalyze the regioselective lactonization of dialdehydes via a bio-Tishchenko-like reaction. The nicotinamide-dependent self-sufficient reduction–oxidation sequence proceeds through a formal intramolecular hydride shift.
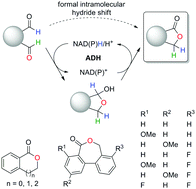
Chem. Commun., 2020,56, 6340-6343
https://doi.org/10.1039/D0CC02509G
Highly regioselective ring-opening of epoxides with amines: a metal- and solvent-free protocol for the synthesis of β-amino alcohols
We herein report a metal- and solvent-free acetic acid-mediated ring-opening reaction of epoxides with amines.
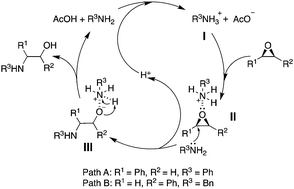
Chem. Commun., 2020,56, 2256-2259
https://doi.org/10.1039/C9CC09048G
Copper-catalyzed asymmetric silyl addition to alkenyl-substituted N-heteroarenes
Asymmetric conjugate addition of PhMe2SiBPin to a wide range of N-heteroaryl alkenes proceeded in the presence of a copper catalyst coordinated with a chiral phosphoramidite ligand to afford useful β-silyl N-heteroarenes in high yields and ees.
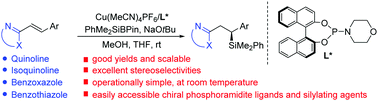
Chem. Commun., 2020,56, 1693-1696
https://doi.org/10.1039/C9CC08910A
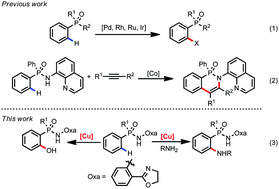
Copper mediated C(sp2)–H amination and hydroxylation of phosphinamides
Copper mediated C(sp2)–H amination and hydroxylation of arylphosphinic acid are accomplished by adopting phosphinamide as the directing group.
Chem. Commun., 2020,56, 1444-1447
https://doi.org/10.1039/C9CC08879B
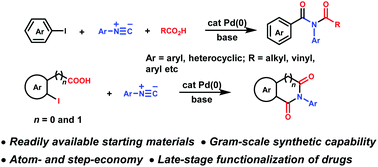
Synthesis of imides via palladium-catalyzed three-component coupling of aryl halides, isocyanides and carboxylic acids
A palladium-catalyzed, three-component synthesis of imides from feedstock aryl halides, carboxylic acids and isocyanides through the intermediacy of isoimide has been developed.
Chem. Commun., 2020,56, 900-903
https://doi.org/10.1039/C9CC08438J
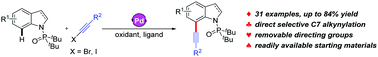
Palladium-catalyzed regioselective C–H alkynylation of indoles with haloalkynes: access to functionalized 7-alkynylindoles
A palladium-catalyzed exclusively selective alkynylation of indoles has been reported, affording concise access to 7-alkynylindoles from readily available starting materials.
Chem. Commun., 2019,55, 13769-13772
https://doi.org/10.1039/C9CC07263B
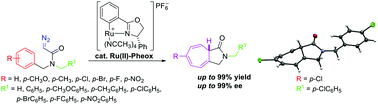
Highly stereoselective intramolecular Buchner reaction of diazoacetamides catalyzed by a Ru(II)–Pheox complex
This work reports the first efficient enantioselective intramolecular Buchner reaction of diazoacetamides.
Chem. Commun., 2019,55, 13398-13401
https://doi.org/10.1039/C9CC06889A
Direct bromocarboxylation of arynes using allyl bromides and carbon dioxide
An unprecedented multicomponent reaction involving arynes, allyl bromides, and CO2 has been developed to construct various allyl o-bromobenzoate scaffolds.

Chem. Commun., 2019,55, 12304-12307
https://doi.org/10.1039/C9CC05495B
A ruthenium-catalyzed free amine directed (5+1) annulation of anilines with olefins: diverse synthesis of phenanthridine derivatives
A ruthenium(II)-catalyzed cross-ring (5+1) annulation between 2-aminobiphenyls and activated olefins is disclosed for succinct synthesis of valuable phenanthridine scaffolds.
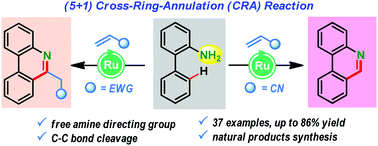
Chem. Commun., 2019,55, 11908-11911
https://doi.org/10.1039/C9CC05717J
Pd-Catalyzed decarboxylative cross-coupling reactions of epoxides with α,β-unsaturated carboxylic acids
A Pd-catalyzed decarboxylative cross-coupling of α,β-unsaturated carboxylic acids with cyclic and acyclic epoxides has been developed.
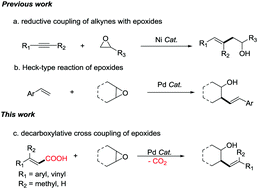
Chem. Commun., 2019,55, 11123-11126
https://doi.org/10.1039/C9CC04795F
Cu-Catalyzed highly selective reductive functionalization of 1,3-diene using H2O as a stoichiometric hydrogen atom donor
A novel high regio- and diastereo-selectivity reductive functionalization of 1,3-diene has been developed using H2O as a stoichiometric hydrogen atom donor.
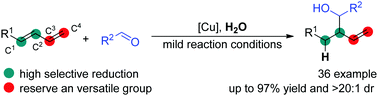
Chem. Commun., 2019,55, 8651-8654
https://doi.org/10.1039/C9CC04011K
A halogen-bonding-catalysed Nazarov cyclisation reaction
Various neutral, mono- and dicationic halogen bond donors were screened for their ability to act as catalysts in a Nazarov cyclisation reaction.
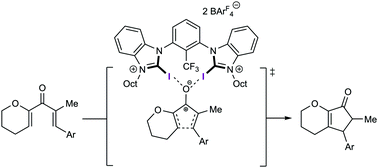
Chem. Commun., 2019,55, 8262-8265
https://doi.org/10.1039/C9CC02816A
Highly active dinuclear cobalt complexes for solvent-free cycloaddition of CO2 to epoxides at ambient pressure
Dinuclear Co-based catalysts are used for the coupling reaction of epoxides and CO2 in the presence of a cocatalyst.

Chem. Commun., 2019,55, 8274-8277
https://doi.org/10.1039/C9CC02626F
Pd-catalyzed synthesis of α,β-unsaturated ketones by carbonylation of vinyl triflates and nonaflates
A general and highly chemoselective Pd-catalyzed protocol for the synthesis of α,β-unsaturated ketones by carbonylation of vinyl triflates and nonaflates is presented. Applying the specific monophosphine ligand cataCXium® A, the synthesis of various vinyl ketones as well as carbonylated natural product derivatives proceeds in good yields.

Chem. Commun., 2019,55, 5938-5941
https://doi.org/10.1039/C9CC02210D
Visible light mediated, metal-free carbene transfer reactions of diazoalkanes with propargylic alcohols
The photolysis of donor–acceptor diazoalkanes in the presence of propargylic alcohols furnishes valuable, sterically demanding tetra-substituted cyclopropenes in high yield under metal-free conditions.
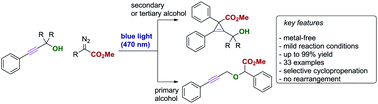
Chem. Commun., 2019,55, 4881-4884
https://doi.org/10.1039/C9CC00927B
Synthesis of polysubstituted 3-aminoindenes via rhodium-catalysed [3+2] cascade annulations of benzimidates with alkenes
A novel Rh-catalysed intermolecular [3+2] cascade cyclization of benzimidates and alkenes has been developed to assemble polysubstituted 3-aminoindenes, which exhibits good functional-group tolerance and excellent regioselectivity.
![Graphical abstract: Synthesis of polysubstituted 3-aminoindenes via rhodium-catalysed [3+2] cascade annulations of benzimidates with alkenes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9CC00567F&imageInfo.ImageIdentifier.Year=2019)
Chem. Commun., 2019,55, 4190-4193
https://doi.org/10.1039/C9CC00567F
Fe(II)-Catalyzed alkenylation of benzylic C–H bonds with diazo compounds
A direct alkenylation of benzylic C(sp3)–H bonds with diazo compounds with FeCl2 as the catalyst and DDQ as the oxidant has been developed.
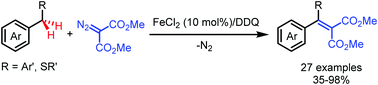
Chem. Commun., 2019,55, 4047-4050
https://doi.org/10.1039/C9CC01060B
Synthesis of dihydroquinolinones via iridium-catalyzed cascade C–H amidation and intramolecular aza-Michael addition
An iridium-catalyzed annulation of chalcones with sulfonyl azides via cascade C–H amidation and aza-Michael addition is developed to provide 2-aryl-2,3-dihydro-4-quinolones.
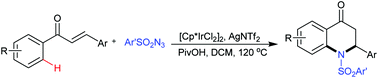
Chem. Commun., 2019,55, 1915-1918
https://doi.org/10.1039/C8CC09751H
Palladium-catalyzed oxidative borylation of conjugated enynones through carbene migratory insertion: synthesis of furyl-substituted alkenylboronates
A new method for the synthesis of furyl-substituted alkenylboronates has been developed by palladium-catalyzed oxidative borylation reaction of conjugated enynones.

Chem. Commun., 2019,55, 59-62
https://doi.org/10.1039/C8CC09024F
Palladium-catalyzed olefination of aryl/alkyl halides with trimethylsilyldiazomethane via carbene migratory insertion
One-pot formation of (E)-vinyl silanes and (E)-silyl-substituted α, β-unsaturated amides could be completed easily via palladium carbene migratory insertion in good yields and high chemoselectivity.
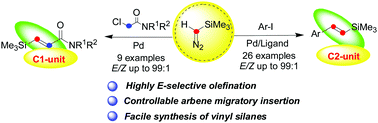
Chem. Commun., 2018,54, 12994-12997
https://doi.org/10.1039/C8CC07664B
An efficient method for retro-Claisen-type C–C bond cleavage of diketones with tropylium catalyst
We report a new convenient and efficient method utilizing the tropylium ion as a mild and environmentally friendly organocatalyst to mediate retro-Claisen-type reactions.
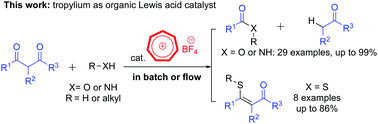
Chem. Commun., 2018,54, 12970-12973
https://doi.org/10.1039/C8CC07329E
Mechanistic and asymmetric investigations of the Au-catalysed cross-coupling between aryldiazonium salts and arylboronic acids using (P,N) gold complexes
Aryldiazonium salts and arylboronic acids were coupled via three different pathways from (P,N)–AuCl complexes, with enantiomeric excesses up to 26%.
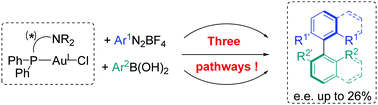
Chem. Commun., 2018,54, 12867-12870
https://doi.org/10.1039/C8CC07530A
Arylation of benzyl amines with aromatic nitriles
The C(sp3)–H arylation of benzyl amines with aromatic nitriles for the synthesis of diarylmethylamines was realized without the assistance of transition-metal and photoirradiation.
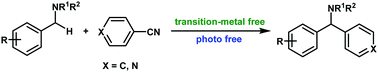
Chem. Commun., 2018,54, 11881-11884
https://doi.org/10.1039/C8CC06408C
C–H functionalisation of aldehydes using light generated, non-stabilised diazo compounds in flow
Here we explore further the use of oxadiazolines, non-stabilised diazo precursors which are bench stable, in direct, non-catalytic, aldehyde C–H functionalisation reactions under UV photolysis in flow and free from additives.

Chem. Commun., 2018,54, 11685-11688
https://doi.org/10.1039/C8CC06202A
Reinventing the De Mayo reaction: synthesis of 1,5-diketones or 1,5-ketoesters via visible light [2+2] cycloaddition of β-diketones or β-ketoesters with styrenes
Taking a different route yields the same product of the De Mayo reaction, but allows the use of visible light instead of UV irradiation.
![Graphical abstract: Reinventing the De Mayo reaction: synthesis of 1,5-diketones or 1,5-ketoesters via visible light [2+2] cycloaddition of β-diketones or β-ketoesters with styrenes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC07044J&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 11602-11605
https://doi.org/10.1039/C8CC07044J
Visible-light photocatalytic bicyclization of β-alkynyl propenones for accessing diastereoenriched syn-fluoren-9-ones
A novel visible-light photocatalytic bicyclization of β-alkynyl propenones with α-bromocarbonyls for highly diastereoselective synthesis of richly decorated syn-fluoren-9-ones is described.
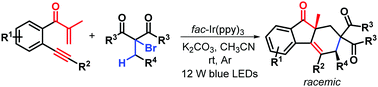
Chem. Commun., 2018,54, 11542-11545
https://doi.org/10.1039/C8CC06086J
Cyanomethyl anion transfer reagents for diastereoselective Corey–Chaykovsky cyclopropanation reactions
A readily available sulfonium salt opens up new synthetic pathways to access nitrile cyclopropanes in a highly diastereoselective fashion.
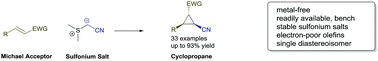
Chem. Commun., 2018,54, 11439-11442
https://doi.org/10.1039/C8CC05602A
Gold-catalyzed annulations of N-aryl ynamides with benzisoxazoles to construct 6H-indolo[2,3-b]quinoline cores
This work reports new annulations of N-aryl ynamides with benzisoxazoles to form 6H-indolo[2,3-b]quinoline derivatives.
![Graphical abstract: Gold-catalyzed annulations of N-aryl ynamides with benzisoxazoles to construct 6H-indolo[2,3-b]quinoline cores](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC04264K&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 10866-10869
https://doi.org/10.1039/C8CC04264K
Asymmetric synthesis of polysubstituted methylenecyclobutanes via catalytic [2+2] cycloaddition reactions of N-allenamides
An asymmetric [2+2] cycloaddition reaction of alkylidene malonates with the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of N-allenamides was developed with a Mg(II)/N,N′-dioxide complex as a catalyst.
C bond of N-allenamides was developed with a Mg(II)/N,N′-dioxide complex as a catalyst.
![Graphical abstract: Asymmetric synthesis of polysubstituted methylenecyclobutanes via catalytic [2+2] cycloaddition reactions of N-allenamides](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC06416D&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 10511-10514
https://doi.org/10.1039/C8CC06416D
Enantioselective acyl-transfer catalysis by fluoride ions
The asymmetric nucleophilic catalysis by fluoride ions at a carbon-based electrophile has been demonstrated for the first time.

Chem. Commun., 2018,54, 10108-10111
https://doi.org/10.1039/C8CC05692G
Reductive cyclisations of amidines involving aminal radicals
The first general study of aminal radical cyclisations, triggered by reduction of amidines with SmI2, delivers quinazolinones with complete diastereocontrol.
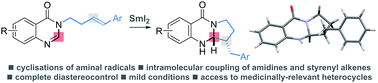
Chem. Commun., 2018,54, 10160-10163
https://doi.org/10.1039/C8CC05178J
Trisubstituted olefin synthesis via Ni-catalyzed hydroalkylation of internal alkynes with non-activated alkyl halides
The stereoselective synthesis of tri-substituted alkenes is challenging.
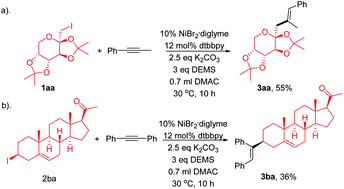
Chem. Commun., 2018,54, 4417-4420
https://doi.org/10.1039/C8CC01577E
Synthesis of bench-stable solid triorganoindium reagents and reactivity in palladium-catalyzed cross-coupling reactions
Triorganoindium reagents can be isolated as bench-stable solid R3In(DMAP) complexes and show excellent reactivity in palladium-catalyzed cross-coupling reactions.
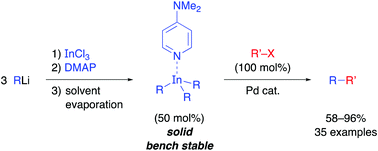
Chem. Commun., 2018,54, 1453-1456
https://doi.org/10.1039/C7CC09344F
Palladium-catalyzed carbene/alkyne metathesis with enynones as carbene precursors: synthesis of fused polyheterocycles
An unprecedented palladium-catalyzed carbene/alkyne metathesis reaction of alkyne-tethered enynones is described, which delivers fused-furans in moderate to good yields.

Chem. Commun., 2018,54, 350-353
https://doi.org/10.1039/C7CC08221E
About this collection
Organic synthesis is far from the level that many people assume. Progress is continuing, but there will not be any dramatic developments. It is more like a glacier that gradually moves forward until it is has finally covered an entire region, but it will still be centuries before synthesis has acquired the status that many people already ascribe to it today.” G. Stork in Nachr. Chem. Techn. 1972, 20, 147. Collated by Antonio Echavarren (ICIQ, Spain), this collection reflects the continuous efforts at gradually moving forward in synthetic methodology.