Mechanistic and asymmetric investigations of the Au-catalysed cross-coupling between aryldiazonium salts and arylboronic acids using (P,N) gold complexes†
Abstract
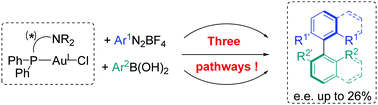
In order to explore the different mechanisms possibly occurring in the Au-catalysed cross-coupling of ArN2BF4 and ArB(OH)2 in the presence of CsF, various stoichiometric experiments were performed on gold complexes with (P,N) ligands. Employing 2-pyridylphenyl-diphenylphosphine allowed us to suggest three different mechanistic pathways, starting either with a transmetallation step, via two consecutive single electron transfers, or by implying a transmetallation between Au(I) and Au(III) species. Moreover, when using commercially available chiral (P,N) ligands, the asymmetric formation of atropoisomeric biaryls from suitable aryldiazonium salts and arylboronic acids could be achieved with e.e. up to 26%.
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...