Reductive cyclisations of amidines involving aminal radicals†
Abstract
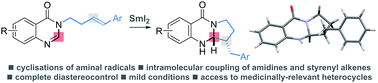
Amidines bearing simple alkenes undergo aminal radical cyclisation upon treatment with SmI2. The mild, reductive electron transfer process delivers medicinally-relevant, polycyclic quinazolinone derivatives in good to excellent yield and typically with complete diastereocontrol.
- This article is part of the themed collections: Organic Synthesis and Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...