Palladium-catalyzed olefination of aryl/alkyl halides with trimethylsilyldiazomethane via carbene migratory insertion†
Abstract
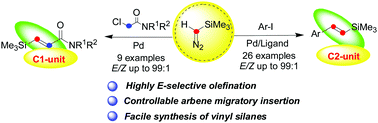
The direct olefination of aryl/alkyl halides with trimethylsilyldiazomethane (TMSD) as a C1- or C2-unit was achieved successfully via a metal carbene migratory insertion process, which offered a new access to afford (E)-vinyl silanes and (E)-silyl-substituted α,β-unsaturated amides in good yields and high chemoselectivity.
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...