Highly regioselective ring-opening of epoxides with amines: a metal- and solvent-free protocol for the synthesis of β-amino alcohols†
Dong
Li
 ,
Jing
Wang
,
Jing
Wang
 ,
Shibo
Yu
,
Shibo
Yu
 ,
Silei
Ye
,
Silei
Ye
 ,
Wenjie
Zou
,
Wenjie
Zou
 ,
Hongbin
Zhang
,
Hongbin
Zhang
 * and
Jingbo
Chen
* and
Jingbo
Chen
 *
*
Key Laboratory of Medicinal Chemistry for Natural Resources, Ministry of Education and Yunnan Province, School of Chemical Science and Technology, Yunnan University Kunming, 650091, P. R. China. E-mail: zhanghb@ynu.edu.cn; chenjb@ynu.edu.cn
First published on 17th January 2020
Abstract
We herein report a metal- and solvent-free acetic acid-mediated ring-opening reaction of epoxides with amines. This process provides β-amino alcohols in high yields with excellent regioselectivity. Importantly, this epoxide ring-opening protocol can be used for the introduction of amines in natural products during late-stage transformations.
β-Amino alcohols are important building blocks for the synthesis of a wide range of biologically active natural and synthetic products, such as natural alkaloids, unnatural amino acids, chiral auxiliaries and pharmaceuticals.1 The most practical and widely used method for preparation of β-amino alcohols is unarguably the ring-opening reaction of epoxides with amines. The direct aminolysis of epoxides, however, usually suffers from lower yields and requires the use of excess amine, long reaction times and hazardous solvents because of the low nucleophilicity of the amines, especially in the cases of aromatic and sterically bulky amines.2 Methods reported in the literature for the epoxide ring-opening reaction with amines are mainly focused on reactions mediated by a range of catalysts, activators and promoters, including silica gel, alumina, zeolite, modified montmorillonite clay,3 ionic liquids,4 solid acids5 and various Lewis acids6 (the vast majority as metal salts) in the presence or absence of a solvent. Although the reactions catalyzed by metal Lewis acids can proceed smoothly to give the β-amino alcohol in good to excellent yields,7 they involve toxic metal salts, and the separation, recovery and recycling of expensive or air-sensitive catalysts is also difficult.
In recent years, metal-free reactions have gained increasing attention in the fine chemical and pharmaceutical industry, and, in most cases, metal-free conditions could completely avoid the above-mentioned shortcomings.8 Solvent-free reactions are also becoming popular because of their environmental benignancy, low cost and low energy consumption.9 Few reports have been published dealing with aminolysis of epoxides under metal- and solvent-free conditions, and the reported examples are limited to liquid epoxides and amines with small molecular weight.10 To the best of our knowledge, the aminolysis of epoxides with amines using carboxylic acid as promoter has not been reported.
Regioselectivity has now become an important focus for the ring-opening reaction of unsymmetrical epoxides, where obtaining one regioisomer exclusively is extremely difficult to achieve. Coates’ work describes some valuable results on a catalyst-controlled regioselective ring opening of unbiased trans-2,3-disubstituted epoxides.6t Islam et al. realized a completely regioselective ring-opening of epoxides with various amines under solvent-free conditions by using a mesoporous TiO2–Fe2O3 mixed oxide material or mesoporous chiral material Fe@SBSAL as a recyclable heterogeneous catalyst.9a,b With trifluoroethanol as reusable promoter and solvent, Heydari et al. provided a facile and efficient synthesis of β-amino alcohols with excellent regioselectivity.10a Most other related methodologies provide a mixture of two regioisomers.
In this paper, we report a metal- and solvent-free ring-opening procedure of symmetrical and unsymmetrical epoxides with various aromatic and aliphatic amines. β-Amino alcohols, the title compounds, can be obtained in high yields with excellent regioselectivity through acetic acid mediated aminolysis of epoxides (see ESI,† Table S2 for a comparison between the performance and regioselectivity of our acetic acid-catalytic study with that of other related reported systems).
To verify the assumption that organic acids would be good promoters for the opening of epoxide rings with amines, we began our investigations by choosing cyclohexene oxide and aniline as the model substrates (Table 1).
| Entry | Catalyst (eq.) | T/°C | Time/min | Yieldb (%) |
|---|---|---|---|---|
| a Reactions were conducted on a 3.05 mmol scale using 1.0 equiv. epoxide, 1.05 equiv. amine. b Isolated yields after column chromatography. | ||||
| 1 | — | rt | 60 | Trace |
| 2 | TfOH (1.0) | rt | 60 | 68 |
| 3 | TsOH (1.0) | rt | 60 | 82 |
| 4 | CF3COOH (1.0) | rt | 60 | 90 |
| 5 | HCOOH (1.0) | rt | 60 | 75 |
| 6 | CH3COOH (1.0) | rt | 60 | 99 |
| 7 | C2H5COOH (1.0) | rt | 60 | 85 |
| 8 | n-C3H7COOH (1.0) | rt | 60 | 76 |
| 9 | CH3COOH (1.0) | 40 | 50 | 99 |
| 10 | CH3COOH (1.0) | 70 | 40 | 99 |
| 11 | CH3COOH (1.0) | 100 | 30 | 98 |
| 12 | CH3COOH (1.0) | rt | 20 | 86 |
| 13 | CH3COOH (1.0) | rt | 40 | 94 |
| 14 | CH3COOH (0.5) | rt | 60 | 83 |
| 15 | CH3COOH (0.1) | rt | 60 | 72 |
In the absence of promoter, only a trace amount of β-amino alcohol was obtained after 1 h reaction at room temperature (Table 1, entry 1). TfOH and TsOH both accelerated this ring-opening reaction (Table 1, entries 2 and 3), but also resulted in the formation of unknown by-products.
Among the carboxylic acids used (Table 1, entries 4–15), trifluoroacetic acid and acetic acid (Table 1, entries 4 and 6) provided the best yields. Considering the low-cost and lack of toxicity, acetic acid was selected as the promoter for further optimization of the reaction conditions. It is reasonable that the pKa of the acidic promoter is positively related to the degree of activation of the epoxide, however, too strong an acidic medium would lead to decomposition of the epoxide and reduce the yield of the β-amino alcohol (see ESI,† Table S1 for a correlation between the pKa and catalytic performance of selected organic acids).
We next optimized the reaction temperature (Table 1, entry 6 and entries 9–11) and the quantity of acetic acid for this reaction (Table 1, entry 6 and entries 14 and 15). Although higher temperature reduced the reaction time, room temperature (ca. 25 °C) is adequate for this reaction. The reaction did not go to completion when less than 1.0 eq. of acetic acid was used (Table 1, entries 14 and 15).
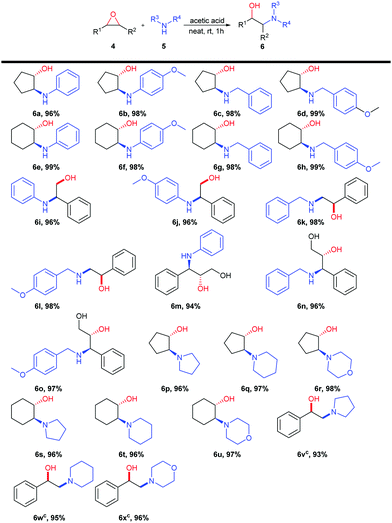
With the optimal reaction conditions in hand (Table 1, entry 6), the generality of this new method was studied using various epoxides and amines (Table 2). To our delight, β-amino alcohols were obtained in excellent yields. High regioselectivities of this reaction were observed for unsymmetrical epoxides. With aliphatic amines being used, the less hindered side of the epoxide rings was attacked to yield secondary alcohols (Table 2, 6k, 6l, 6v, 6w and 6x). The regioselectivities were reversed when aromatic amines were used (Table 2, 6i and 6j, Scheme 1). Because of the presence of a neighboring hydroxyl group, trans-2,3-epoxycinnamyl alcohol provided β-amino alcohols by opening the benzylic position of the epoxide (Table 2, 6m–o) with both aliphatic and aromatic amines (see ESI,† Fig. S1 for a plausible mechanism for this regioselectivity).
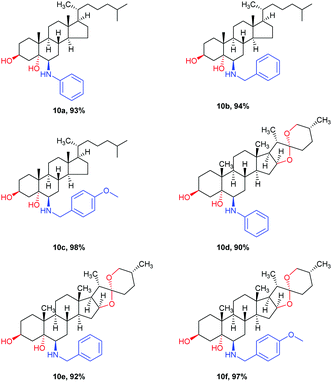
Encouraged by the successful results described above, we next extended our metal- and solvent-free method for the ring-opening reactions of sterically hindered steroidal epoxides, with the aim of synthesizing biologically active aminosteroids. It has always been an issue for solvent-free reactions involving a solid-state reactant with high melting point because of the poor mixing effect. Fortunately, a gel-state emerged when the steroidal 5α,6α-epoxide (7) mixed with (4-methoxyphenyl) methanamine and acetic acid upon heating to 60–150 °C. Optimizing the reaction temperature and the amount of acetic acid (Table 3) provided the best conditions, namely 4.0 equivalents of acetic acid and stirring at 150 °C for 2 hours. The target product was obtained in 98% yield (Table 3, entry 7). Several substrates were tested under the optimized conditions and the results are summarized in Table 4.
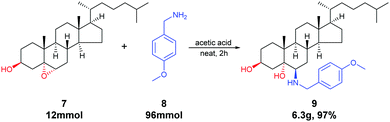
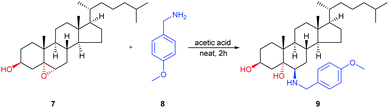
We further explored the scalability and demonstrated the synthetic value of this method through the gram-scale synthesis of 6β-aminosteroid (9). The epoxide 7 (5.0 g, 0.012 mol) was combined with amine 8 (13.3 g, 0.096 mol) and acetic acid (3.0 g, 0.048 mol), heating at 150 °C for 2 h, followed by workup and purification on silica gel column chromatography afforded 6.3 g of 9 (97% yield, Scheme 1).
As seen from Fig. 1, the true catalytic species is the nitrogen-onium ion (I) formed by acetic acid and amine. In path A, the more acidic I was derived from weakly basic PhNH2. Subsequent bonding between I and styrene oxide generated the transition state II, from which a differential polarization of the benzylic carbon and the terminal carbon of the epoxide took place and favored the selective nucleophilic attack at the benzylic position by weakly nucleophilic PhNH2. In path B, BnNH2 was a relatively stronger base, making nitrogen-onium ion (I) less acidic and exerting a weak activation of the epoxide (II). In addition, the relatively higher nucleophilicity of BnNH2 favored nucleophilic attack at the terminal carbon atom of the styrene oxide.
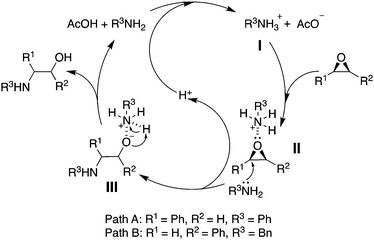 | ||
| Fig. 1 Plausible reaction pathway for the regioselective acetic acid mediated ring-opening reaction of epoxides with amines. | ||
In summary, we have developed a novel metal- and solvent-free protocol for the preparation of β-amino alcohols by acetic acid-mediated aminolysis of epoxides. The less hindered epoxides can react with aliphatic and aromatic amines at room temperature, while the solid-state sterically hindered epoxides could be reacted at higher temperatures to provide the products in excellent yields. Furthermore, this method enables the efficient synthesis of a variety of β-amino alcohols with excellent and tunable regioselectivity. It is noteworthy that this synthetic protocol is environmentally benign and could be used for the preparation of β-amino alcohols on a gram-scale, which is crucial in the fine-chemical and pharmaceutical industries.
We acknowledge the support from the National Natural Science Foundation of China (21662041, U1702286), the Yunnan Provincial Science & Technology department (2018FA045), the Program for Changjiang Scholars and Innovative Research Teams in Universities (IRT17R94), The Project of Innovative Research Team of Yunnan Province to W. L. Xiao.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) K. C. Nicolaou and C. N. Boddy, J. Am. Chem. Soc., 2002, 124, 10451 CrossRef CAS PubMed; (b) C. W. Johannes, M. S. Visser, G. S. Weatherhead and A. H. Hoveyda, J. Am. Chem. Soc., 1998, 120, 8340 CrossRef CAS; (c) D. A. Alonso, D. Guijarro, P. Pinho, O. Temme and P. G. Andersson, J. Org. Chem., 1998, 63, 2749 CrossRef CAS PubMed; (d) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835 CrossRef CAS PubMed; (e) B. L. Chng and A. Ganesan, Bioorg. Med. Chem. Lett., 1997, 7, 1511 CrossRef CAS; (f) E. J. Corey and F. Y. Zhang, Angew. Chem., Int. Ed., 1999, 38, 1931 CrossRef CAS; (g) G. Li, H. T. Chang and K. B. Sharpless, Angew. Chem., Int. Ed. Engl., 1996, 35, 451 CrossRef CAS; (h) P. Castejon, A. Moyano, M. A. Pericas and A. Riera, Tetrahedron, 1996, 52, 7063 CrossRef CAS.
- J. A. Deyrup and C. L. Moyer, J. Org. Chem., 1969, 34, 175 CrossRef CAS.
- (a) A. K. Chakraborti, S. Rudrawar and A. Kondaskar, Org. Biomol. Chem., 2004, 2, 1277 RSC; (b) T. Hashiyama, H. Inoue, M. Takeda, S. Murata and T. Nagao, Chem. Pharm. Bull., 1985, 33, 2348 CrossRef CAS; (c) W. C. Lumma Jr and J. P. Springer, J. Org. Chem., 1981, 46, 3735 CrossRef; (d) R. I. Kureshy, S. Singh, N. H. Khan, S. H. R. Abdi, E. Suresh and R. V. Jasra, J. Mol. Catal. A: Chem., 2007, 264, 162 CrossRef CAS; (e) B. Diganta, S. Lakshi and K. D. Dipak, Appl. Catal., A, 2014, 487, 195 CrossRef.
- (a) J. S. Yadav, B. V. S. Reddy, A. K. Basak and A. V. Narsaiah, Tetrahedron Lett., 2003, 44, 1047 CrossRef CAS; (b) S. V. Malhotra, R. P. Andal and V. Kumar, Synth. Commun., 2008, 38, 4160 CrossRef CAS.
- (a) S. R. Kumar and P. Leelavathi, J. Mol. Catal. A: Chem., 2007, 266, 65 CrossRef CAS; (b) M. Vijender, P. Kishore, P. Narender and B. Satyanarayana, J. Mol. Catal. A: Chem., 2007, 266, 290 CrossRef CAS; (c) N. Azizi and M. R. Saidi, Tetrahedron, 2007, 63, 888 CrossRef CAS; (d) A. Kamal, B. R. Prasad, A. M. Reddy and M. N. A. Khan, Catal. Commun., 2007, 8, 1876 CrossRef CAS; (e) Y. L. N. Murthy, B. S. Diwakar and B. Govindh, Chem. Sci. Trans., 2013, 2, 805 CAS; (f) A. K. Shah, M. Kumar, S. H. R. Abdi, R. I. Kureshy, N. H. Khan and H. C. Bajaj, Appl. Catal., A, 2014, 486, 105 CrossRef CAS.
- (a) M. C. Singh and R. K. Peddinti, Tetrahedron Lett., 2007, 48, 7354 CrossRef CAS; (b) S. R. Kumar and P. Leelavathi, J. Mol. Catal. A: Chem., 2007, 266, 65 CrossRef CAS; (c) S. Kobayashi, M. Sugiura, H. Kitagawa and W. W. Lam, Chem. Rev., 2002, 102, 2227 CrossRef CAS PubMed; (d) G. Sundararajan, K. Vijayakrishnaa and B. Varghese, Tetrahedron Lett., 2004, 45, 8253 CrossRef CAS; (e) A. K. Chakraborti, S. Rudrawar and A. Kondaskar, Org. Biomol. Chem., 2004, 2, 1277 RSC; (f) G. Sabitha, G. S. K. K. Reddy, K. B. Reddy and J. S. Yadav, Synthesis, 2003, 2298 CrossRef CAS; (g) N. R. Swamy, T. V. Goud, S. M. Reddy, P. Krishnaiah and Y. Venkateswarlu, Synth. Commun., 2004, 34, 727 CrossRef CAS; (h) D. Thibeault and D. Poirier, Synlett, 2003, 1192 CAS; (i) E. Mai and C. Schneider, Chem. – Eur. J., 2007, 13, 2729 CrossRef CAS PubMed; (j) G. Hattori, A. Yoshida, Y. Miyake and Y. Nishibayashi, J. Org. Chem., 2009, 74, 7603 CrossRef CAS PubMed; (k) A. Procopio, M. Gaspari, M. Nardi, M. Oliverio and O. Rosati, Tetrahedron Lett., 2008, 49, 2289 CrossRef CAS; (l) T. Ollevier and E. Nadeau, Tetrahedron Lett., 2008, 49, 1546 CrossRef CAS; (m) J. S. Yadav, A. R. Reddy, A. V. Narsaiah and B. V. S. Reddy, J. Mol. Catal. A: Chem., 2007, 261, 207 CrossRef CAS; (n) A. R. Khosropour, M. M. Khodaei and K. Ghozati, Chem. Lett., 2004, 33, 304 CrossRef CAS; (o) A. K. Chakraborti and A. Kondaskar, Tetrahedron Lett., 2003, 44, 8315 CrossRef CAS; (p) A. K. Chakraborti, A. Kondaskar and S. Rudrawar, Tetrahedron, 2004, 60, 9085 CrossRef CAS; (q) A. K. Chakraborti, S. Rudrawar and A. Kondaskar, Eur. J. Org. Chem., 2004, 3597 CrossRef CAS; (r) D. E. Frantz, R. Fässler and E. M. Carreira, J. Am. Chem. Soc., 2000, 122, 1806 CrossRef CAS; (s) F. Fringuelli, F. Pizzo, S. Tortoioli and L. Vaccaro, J. Org. Chem., 2004, 69, 7745 CrossRef CAS PubMed; (t) M. Lee, J. R. Lamb, M. J. Sanford, A. M. LaPointe and G. W. Coates, Chem. Commun., 2018, 54, 12998 RSC.
- (a) J. Agarwal, A. Duley, R. Ran and R. K. Peddinti, Synthesis, 2009, 2790 CAS; (b) N. Tan, S. Yin, Y. Li, R. Qiu, Z. Meng, X. Song, S. Luo, C. T. Au and W. Y. Wong, J. Organomet. Chem., 2011, 696, 1579 CrossRef CAS.
- (a) N. K. Shee, F. A. O. Adekunle, D. Das, M. G. B. Drew and D. Datta, Inorg. Chim. Acta, 2011, 375, 101 CrossRef CAS; (b) G. G. Deng, M. Y. Li, K. L. Yu, C. X. Liu, Z. F. Liu, S. Z. Duan, W. Chen, X. D. Yang, H. B. Zhang and P. J. Walsh, Angew. Chem., Int. Ed., 2019, 58, 2826 CrossRef CAS PubMed.
- (a) S. Roy, B. Banerjee, N. Salam, A. Bhaumik and S. M. Islam, ChemCatChem, 2015, 7, 2689 CrossRef CAS; (b) S. Roy, P. Bhanja, S. S. Islam, A. Bhaumik and S. M. Islam, Chem. Commun., 2016, 52, 1871 RSC; (c) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (d) K. V. Katkar, P. S. Chaudhari and K. G. Akamanchi, Green Chem., 2011, 13, 835 RSC; (e) U. M. Lindstrom, Chem. Rev., 2002, 102, 2751 CrossRef PubMed; (f) M. C. Pirrung, Chem. – Eur. J., 2006, 12, 1312 CrossRef CAS PubMed; (g) H. Y. Zeng, Z. M. Wang and C. J. Li, Angew. Chem., Int. Ed., 2019, 58, 2859 CrossRef CAS PubMed.
- (a) S. Khaksar, A. Heydari, M. Tajbakhsh and H. R. Bijanzadeh, J. Fluorine Chem., 2010, 131, 106 CrossRef CAS; (b) D. Soto-Castro, R. C. L. Contreras, M. Pina-Canseco, R. Santillán, M. T. Hernández-Huerta, G. E. Negrón Silva, E. Pérez-Campos and S. Rincón, Steroids, 2017, 126, 92 CrossRef CAS PubMed; (c) M. R. Acocella, L. D'Urso, M. Maggio and G. Guerra, ChemCatChem, 2016, 8, 1915 CrossRef CAS; (d) A. Kamal, B. R. Prasad, A. M. Reddy and M. N. A. Khan, Catal. Commun., 2007, 8, 1876 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc09048g |
| This journal is © The Royal Society of Chemistry 2020 |