Synthesis of dihydroquinolinones via iridium-catalyzed cascade C–H amidation and intramolecular aza-Michael addition†
Abstract
An iridium-catalyzed annulation of chalcones with sulfonyl azides via cascade C–H amidation and intramolecular aza-Michael addition was developed, affording a variety of 2-aryl-2,3-dihydro-4-quinolones in moderate to good yields. This reaction features easy operation, readily available starting materials, and the cascade formation of two C–N bonds in one pot.
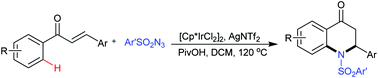
- This article is part of the themed collection: Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...