Combination of organocatalytic oxidation of alcohols and organolithium chemistry (RLi) in aqueous media, at room temperature and under aerobic conditions†
David
Elorriaga‡
 a,
María Jesús
Rodríguez-Álvarez‡
a,
Nicolás
Ríos-Lombardía
b,
Francisco
Morís
b,
Alejandro
Presa Soto
a,
María Jesús
Rodríguez-Álvarez‡
a,
Nicolás
Ríos-Lombardía
b,
Francisco
Morís
b,
Alejandro
Presa Soto
 a,
Javier
González-Sabín
a,
Javier
González-Sabín
 *b,
Eva
Hevia
*b,
Eva
Hevia
 *c and
Joaquín
García-Álvarez
*c and
Joaquín
García-Álvarez
 *a
*a
aDepartamento de Química Orgánica e Inorgánica, (IUQOEM) Facultad de Química, Universidad de Oviedo, E-33071, Oviedo, Spain. E-mail: garciajoaquin@uniovi.es
bEntreChem SL. Santo Domingo de Guzmán, 33011, Oviedo, Spain
cDepartment für Chemie und Biochemie, Universität Bern, CH3012, Bern, Switzerland
First published on 30th June 2020
Abstract
A tandem protocol to access tertiary alcohols has been developed which combines the organocatalytic oxidation of secondary alcohols to ketones followed by their chemoselective addition by several RLi reagents. Reactions take place at room temperature, under air and in aqueous solutions, a trio of conditions that are typically forbidden in polar organometallic chemistry.
As Nature's solvent in biological processes, the use of water as a medium in organic synthesis1 is widely recognized as an important strategy towards developing sustainable chemical processes that implement some of the most significant Principles of Green Chemistry.2 Water is abundant, non-toxic, cost-effective, non-flammable, renewable, and non-volatile3 and it has already shown an enormous potential as a versatile reaction medium in many cornerstone organic transformations such as oxidations, reductions, reductive aminations and pericyclic reactions to name just a few,1 as well as in a myriad of transition-metal-catalyzed reactions.4 Furthermore, in many cases water is not only a greener alternative to toxic organic solvents but can also be a superior medium, improving reactivities and selectivities, simplifying work up procedures and facilitating catalyst recycling.5 Nevertheless, despite these key advantages, until recently, water has shown very little promise as a solvent to carry out organolithium chemistry with only a few examples in the literature.6 This is probably as a consequence of the common assumption that these polar reagents rapidly decompose in contact with this solvent. In fact, the use of protective inert atmosphere conditions and stringently dry organic solvent along with strict control of the reaction temperatures (usually of the order of −78 °C) are usually mandatory to avoid fast degradation and control the exceptionally high reactivity of organolithium reagents.7 However, what was thought impossible just a few years ago, an aerobic sustainable polar organometallic chemistry, is now starting to look distinctly possible.6,8 Thus, Capriati has reported the chemoselective addition of organolithiums to carbonyl compounds8d and to nitriles and imines8h under on water conditions as well as palladium catalyzed direct cross-couplings of RLi and hetero(aryl) halides.8i Furthermore bioinspired Deep Eutectic Solvents (DESs) which are usually made up of a combination of biorenewable hydrogen bond acceptors [e.g., ammonium salts like choline chloride (ChCl)] and hydrogen bond donor (e.g., water, glycerol)9 have also shown great promise as non-conventional media for air and moisture compatible organolithium synthesis as demonstrated recently by our groups for nucleophilic arylation/alkylation of ketones, imines and nitriles10 as well as anionic polymerization of styrenes.11,12 Remarkable, in some of these examples, higher yields and selectivities were achieved than when using inert atmosphere protocols. Expanding even further the synthetic applications of polar organometallic chemistry in water, herein we report a new protocol that pairs these operational breakthroughs with an organocatalytic transformation, uncovering a novel one-pot tandem approach to access highly-substituted tertiary alcohols.
By eliminating the need of isolation and purification of reaction intermediates (and subsequently minimizing chemical waste generation, reaction times and energy consumption), one-pot tandem processes are highly useful and efficient green approaches in modern synthesis for the production of organic molecules.13 More often than not, these protocols tend to use the same type of transformational tools (metal-, main-group element-, bio- or organo-catalysts) throughout all the tandem process, whereas the number of examples which combine different methodologies is scarce, especially those that also employ sustainable solvents. In this regard recently we have successfully combined transition-metal-catalysis in water or DESs with (Scheme 1a): (i) chemoselective addition of organolithium reagents (RLi) to ketones as transient intermediates;14 and (ii) enzymatic enantioselective bioamination (transaminases, ATA) or bioreduction (ketoreductases, KRED) of in situ generated prochiral ketones.15 Breaking new ground in this evolving field, we combine for the first time organocatalysis and organolithium chemistry in water as a new methodology to prepare highly substituted tertiary alcohols. The approach employed comprises the organo-catalyzed oxidation of secondary alcohols (furnishing ketones in situ as intermediates) followed by subsequent chemoselective and ultrafast addition of organolithium reagents affording the relevant tertiary alcohols. Reactions were carried out under one-pot conditions in aqueous media, under air and at room temperature (Scheme 1b).
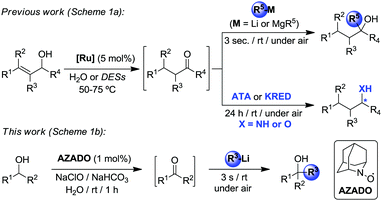 | ||
| Scheme 1 Design of one-pot tandem combinations that rely on different organic synthetic tools in water or DESs. | ||
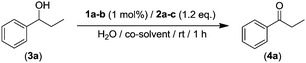
Building on previous studies reported by some of us, in which we have uncovered the efficient organocatalytic activity of AZADO (2-azaadamantane N-oxyl, 1a) in combination with an aqueous solution of NaClO (2a, acting as external oxidant) as a straightforward and operationally simple method for the selective oxidation of secondary alcohols;16 we selected, as a model reaction, the oxidation of the benzylic-type alcohol 3a (see Table 1). Firstly, we assessed if the use of toxic organic co-solvents such as PhCF3, CH2Cl2 or CH3CN was actually required in order to achieve quantitative yields of the desired ketone 4a (entries 1–3, Table 1).16
| Entry | Organocat. | Oxidant | Co-solvent | Yieldb (%) |
|---|---|---|---|---|
| a General reaction conditions are described in ESI. b Isolated yields. | ||||
| 1 | AZADO (1a) | aq. NaClO (2a) | PhCF3 | 95 |
| 2 | AZADO (1a) | aq. NaClO (2a) | CH2Cl2 | 99 |
| 3 | AZADO (1a) | aq. NaClO (2a) | CH3CN | 99 |
| 4 | AZADO (1a) | aq. NaClO (2a) | — | 99 |
| 5 | AZADO (1a) | KMnO4 (2b) | — | 69 |
| 6 | AZADO (1a) | I2 (2c) | — | 13 |
| 7 | Minoxidil (1b) | aq. NaClO (2a) | — | 17 |
| 8 | Minoxidil (1b) | KMnO4 (2b) | — | 42 |
| 9 | Minoxidil (1b) | I2 (2c) | — | 4 |
Pleasingly, we found that 1-phenylpropan-1-ol (3a) can be quantitatively converted into the desired propiophenone (4a, entry 4, Table 1) under the same reaction conditions (1 h, rt, 270 mM loading of substrate) in the absence of these toxic organic co-solvents. For completeness of this parametrization, we also studied the use of other co-oxidants for AZADO (1a) like KMnO4 (2b, entry 5, Table 1) or I2 (2c, entry 6, Table 1) finding in both cases reduced activity (69–13%). Interestingly, we found that when AZADO was replaced by a different amine N-oxide such as minoxidil (1b, 6-piperidin-1-ylpyrimidine-2,4-diamine 3-oxide) in combination with the aforementioned sacrificial oxidants (NaClO, entry 7; KMnO4, entry 8; and I2, entry 9; Table 1) significant lower conversions were observed (4–42% yields), highlighting the high efficiency of the organocatalytic/oxidation NaClO/AZADO system while operating in aqueous media.
Encouraged by these initial results, which show that we can quantitatively prepare ketones from secondary alcohols in aqueous media at room temperature and in the absence of any organic co-solvents, we next pondered if this transformation could also be part of a modular combination, where the relevant synthesized ketones could subsequently undergo chemoselective alkylation using a one-pot approach while operating in aqueous media under air. Towards this target, it should be noted that s-block polar organometallic reagents such as Grignard (RMgX) and organolithium (RLi) reagents are widely used for addition reactions to ketones, being a fundamental and versatile method to generate C–C bonds to access tertiary alcohols.17 Notwithstanding, the chemoselectivity of these processes can be compromised by the formation of undesired reduction and/or enolization products, resulting from competing β-hydride elimination and deprotonation reactions respectively.18 Recently our groups have shown using DESs which combine ammonium salt choline chloride (ChCl) with water or glycerol (Gly) as reaction media, Grignard and organolithium reagents can promote room temperature chemoselective ketone alkylation/arylation reactions.10 Along with greater selectivities and milder reaction conditions, these reactions are compatible with the presence of air.10 However, DESs are not compatible with the organocatalytic oxidation system AZADO/NaClO, as the addition of NaClO to the eutectic reaction media produced the formation of Cl2 gas through the expected comproportionation reaction between the Cl− and ClO− anions.
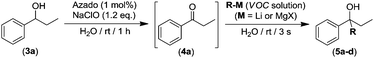
We next assessed whether this tandem oxidation/alkylation approach could be compatible with aqueous media. Thus, we selected as a model reaction the AZADO/NaClO organocatalyzed oxidation of 1-phenylpropan-1-ol (3a) into ketone 4a followed by the direct addition of n-BuLi (Table 2). Firstly, the reaction mixture containing the alcohol 3a (270 mM) and the organocatalytic oxidation system (AZADO/NaClO) was allowed to react in aqueous media, at room temperature and in the presence of air for 1 h to trigger the expected oxidation. As soon as the total conversion of 3a into propiophenone (4a) was observed (1 h, GC analysis), n-BuLi was directly added to the reaction mixture (without any intermediate step of isolation or purification of ketone 4a), in the absence of protecting atmosphere and at room temperature (conditions usually forbidden for RLi reagents).17 Astonishing, the almost instantaneous formation (3 s) of the relevant tertiary alcohol 5a was observed, finding a significantly improved yield of 76% when three equivalents of the organolithium reagent were employed (entry 4, Table 2).19 However, gradually decreasing the number of equivalents of n-BuLi (from 3 to 1) produces a concomitant diminution of the nucleophilic addition of n-BuLi into the in situ formed ketone 4a, from 76 to 23% yield of the final tertiary alcohol 5a (entries 1–4, Table 2). At this point, it is important to note that in all cases (entries 1–4, Table 2) the one-pot tandem construction of the tertiary alcohol 5a occurred with total chemoselectivity, as no side products were observed in the crude reaction mixture (containing only the desired final product 5a and the unreacted ketone 4a). We next decided to expand the scope to other organolithium (RLi) or organomagnesium (RMgX) reagents.
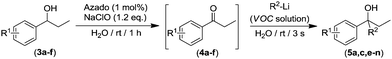
Interestingly we found that other aliphatic organolithium reagents such as MeLi (entry 5, Table 2); sec-BuLi (entry 6, Table 2) can selectively add across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond of 4a under the above mentioned conditions, affording alcohols 5b and 5c in excellent yields (70 and 83% respectively) whereas using less nucleophilic PhLi, 5d is obtained in a 35% yield (entry 6, Table 2). The high conversions and chemoselectivities observed while operating at room temperature are particularly noteworthy for sec-BuLi which has a greater tendency to undergo β-hydride elimination, and has to be usually stored at low temperatures (2–8 °C) to avoid degradation via this side reaction. Furthermore, Ishihara has shown that when n-BuLi is employed as an alkylating reagent towards enolizable ketones variable amounts of the aldol condensation products are obtained even when operating at −78 °C in THF under strict inert atmosphere conditions.18b On the other hand, this method does not seem compatible with Grignard reagents and when n-BuMgCl was employed as an alkylating reagent, alcohol 5a could not be detected (entry 8, Table 2). This lack of reactivity is consistent with previous studies in the addition of Grignard reagents to carbonyl compounds in water that have shown that n-BuMgCl undergoes complete protonation in competition with carbonyl addition.20 At this point we would like to highlight the use of commercially available solutions of RMgX and RLi reagents containing different organic solvents, namely hexane for n-Buli, diethyl ether for MeLi, and dibutyl ether for PhLi. We hypothesize that besides the intrinsic reactivity of each reagent, both the nature and the amount of the organic accompanying solvent is critical for the outcome of the addition reaction, based on the premise that the reactions are taking place under “on water” conditions.21 Accordingly, the more water-immiscible the RLi solvent and the smaller concentration of the commercially available organolithium solution, the more favoured that interface and therefore the better performance. In good agreement, the highest yield was obtained in cyclohexane (sec-BuLi, entry 6), the most hydrophobic solvent of the series and with the smaller concentration (1.4 M), followed by hexane (n-BuLi, 1.6 M, entry 4) and finally the ether solvent (entries 5 and 7). In the same line, another interesting finding was that when acetophenone was reacted with n-BuLi using water as a reaction medium under exactly the same conditions depicted in Table 2, the alkylation reaction is suppressed. Whereas, with propiophenone (4a), alcohol 5a was obtained in a 76%. A plausible explanation could be the different solubilities in water of both ketones, with acetophenone showing a greater solubility in water than 4a, again in agreement with the possibility that these addition reactions are taking place under “on water” conditions.21 “On water” reactions are thought to occur at the organic/liquid water interface with water insoluble reactants. In this regard Capriati et al. have recently postulated that interfacial H-bonding to water insoluble carbonyl compounds embedded at the organic/water interphase may play a key role in favouring “on-water” nucleophilic additions promoted by RMgX and RLi reagents.8d,h Once the scope of the s-block highly-polar organometallic reagents was established, we decided to further expand this one-pot tandem oxidation/RLi addition in aqueous media to a series of secondary alcohols (3a–f, Table 3) and n-BuLi or sec-BuLi as alkylating reagents.
O double bond of 4a under the above mentioned conditions, affording alcohols 5b and 5c in excellent yields (70 and 83% respectively) whereas using less nucleophilic PhLi, 5d is obtained in a 35% yield (entry 6, Table 2). The high conversions and chemoselectivities observed while operating at room temperature are particularly noteworthy for sec-BuLi which has a greater tendency to undergo β-hydride elimination, and has to be usually stored at low temperatures (2–8 °C) to avoid degradation via this side reaction. Furthermore, Ishihara has shown that when n-BuLi is employed as an alkylating reagent towards enolizable ketones variable amounts of the aldol condensation products are obtained even when operating at −78 °C in THF under strict inert atmosphere conditions.18b On the other hand, this method does not seem compatible with Grignard reagents and when n-BuMgCl was employed as an alkylating reagent, alcohol 5a could not be detected (entry 8, Table 2). This lack of reactivity is consistent with previous studies in the addition of Grignard reagents to carbonyl compounds in water that have shown that n-BuMgCl undergoes complete protonation in competition with carbonyl addition.20 At this point we would like to highlight the use of commercially available solutions of RMgX and RLi reagents containing different organic solvents, namely hexane for n-Buli, diethyl ether for MeLi, and dibutyl ether for PhLi. We hypothesize that besides the intrinsic reactivity of each reagent, both the nature and the amount of the organic accompanying solvent is critical for the outcome of the addition reaction, based on the premise that the reactions are taking place under “on water” conditions.21 Accordingly, the more water-immiscible the RLi solvent and the smaller concentration of the commercially available organolithium solution, the more favoured that interface and therefore the better performance. In good agreement, the highest yield was obtained in cyclohexane (sec-BuLi, entry 6), the most hydrophobic solvent of the series and with the smaller concentration (1.4 M), followed by hexane (n-BuLi, 1.6 M, entry 4) and finally the ether solvent (entries 5 and 7). In the same line, another interesting finding was that when acetophenone was reacted with n-BuLi using water as a reaction medium under exactly the same conditions depicted in Table 2, the alkylation reaction is suppressed. Whereas, with propiophenone (4a), alcohol 5a was obtained in a 76%. A plausible explanation could be the different solubilities in water of both ketones, with acetophenone showing a greater solubility in water than 4a, again in agreement with the possibility that these addition reactions are taking place under “on water” conditions.21 “On water” reactions are thought to occur at the organic/liquid water interface with water insoluble reactants. In this regard Capriati et al. have recently postulated that interfacial H-bonding to water insoluble carbonyl compounds embedded at the organic/water interphase may play a key role in favouring “on-water” nucleophilic additions promoted by RMgX and RLi reagents.8d,h Once the scope of the s-block highly-polar organometallic reagents was established, we decided to further expand this one-pot tandem oxidation/RLi addition in aqueous media to a series of secondary alcohols (3a–f, Table 3) and n-BuLi or sec-BuLi as alkylating reagents.
| Entry | R1 | R2-M | Product | Yieldb (%) |
|---|---|---|---|---|
| a General reaction conditions are described in ESI. b Isolated yields. | ||||
| 1 | H (3a) | n-BuLi | 5a | 76 |
| 2 | H (3a) | sec-BuLi | 5c | 83 |
| 3 | p-Cl (3b) | n-BuLi | 5e | 82 |
| 4 | p-Cl (3b) | sec-BuLi | 5f | 54 |
| 5 | p-Br (3c) | n-BuLi | 5g | 95 |
| 6 | p-Br (3c) | sec-BuLi | 5h | 73 |
| 7 | p-Me (3d) | n-BuLi | 5i | 90 |
| 8 | p-Me (3d) | sec-BuLi | 5j | 66 |
| 9 | p-MeO (3e) | n-BuLi | 5k | 40 |
| 10 | p-MeO (3e) | sec-BuLi | 5l | 74 |
| 11 | o-Me (3f) | n-BuLi | 5m | 68 |
| 12 | o-Me (3f) | sec-BuLi | 5n | 39 |
We found that this method tolerates a variety of functional groups in the aromatic scaffold of the starting alcohols 3a–f being compatible with the presence of either electron-withdrawing (3b–c, entries 3–6) or electron-donating groups (3d–f, entries 7–12) giving rise to the desired tertiary alcohols 5a,c,e–n in good yields (up to 95%), and without the need of any halfway step of isolation or purification of the intermediate ketones 4a–f, which were always formed in quantitative yields after 1 h of reaction (GC analysis). In general, better yields of the final tertiary alcohols were obtained when n-BuLi (even entries in Table 3) was used as alkylating reagent. This experimental observation is in good agreement with the expected lower nucleophilic activity of the more sterically demanding sec-BuLi when compared with linear n-BuLi.17 At this point, it is important to note that although electronic properties of the substituents in the aromatic ring have only a modest effect, the presence of an ortho-substituent (entries 11 and 12, Table 3) produces a considerable decrease in the yield of the final alcohols 5m–n. This experimental observation seems to indicate that the electronic effects have a smaller impact than steric hindrance which can be associated to the virtually instantaneous nature of the addition reactions in aqueous media. Interestingly, other possible competing processes with the addition reaction or RLi to ketones like: (i) metalation of benzylic position in substrates 3d–f; or (ii) Li-halogen exchange processes in alcohols 3b–c, were not detected.
In conclusion, edging closer towards the development of air and water compatible organolithium chemistry, these findings have uncovered the potential of using water as an alternative sustainable reaction medium for tandem protocols that combine organocatalysis with the use of organolithium reagents. Interestingly water not only enables more environmentally benign reaction conditions (under air, room temperature) but also greater selectivities than when using conventional organic solvents under inert atmosphere conditions.
We thank the MINECO (projects CTQ2017-88357-P, CTQ2016-81797-REDC and CTQ2016-75986-P). J. G.-A. thanks PhosAgro, UNESCO and IUPAC for the award of a “Green Chemistry for Life Grant”. EH thanks the University of Bern for the generous sponsorship of this research. D. E. thanks Gobierno del Principado de Asturias and EU for the award of a postdoctoral fellowship.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. C. Hailes, Org. Proces Res. Dev., 2007, 11, 114 CrossRef CAS; (b) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- (a) T. W. Seow, C. K. Lim, M. H. M. Nor, M. F. M. Mubarak, C. Y. Lam, A. Yahya and Z. Ibrahim, Int. J. Appl. Environ. Sci., 2016, 11, 111 Search PubMed; (b) C. A. Martínez-Huitle and S. Ferro, Chem. Soc. Rev., 2006, 35, 1324 RSC.
- T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679 CrossRef CAS PubMed.
- M. O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC.
- (a) J. García-Álvarez, E. Hevia and V. Capriati, Eur. J. Org. Chem., 2015, 6779 CrossRef; (b) J. García-Álvarez, E. Hevia and V. Capriati, Chem. – Eur. J., 2018, 24, 14854 CrossRef PubMed.
- (a) V. Capriati, F. M. Perna and A. Salomone, Dalton Trans., 2014, 43, 14204 RSC; (b) U. Wietelmann and J. Klett, Z. Anorg. Allg. Chem., 2018, 644, 194 CrossRef CAS PubMed.
- (a) T. Laube, J. D. Dunitz and D. Seebach, Helv. Chim. Acta, 1985, 68, 1373 CrossRef CAS; (b) P. G. Cozzi and L. Zoli, Angew. Chem., Int. Ed., 2008, 47, 4162 CrossRef CAS PubMed; (c) V. Juste-Navarro, I. Delso, T. Tejero and P. Merino, Chem. – Eur. J., 2016, 22, 11527 CrossRef CAS PubMed; (d) L. Cicco, S. Sblendorio, R. Mansueto, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Sci., 2016, 7, 1192 RSC; (e) D. Seebach, Isr. J. Chem., 2017, 57, 55 CrossRef CAS; (f) Y. Gimbert, D. Lesage, C. Fressigné and J. Maddaluno, J. Org. Chem., 2017, 82, 8141 CrossRef CAS PubMed; (g) I. Koehne, S. Bachmann, R. Herbst-Irmer and D. Stalke, Angew. Chem., Int. Ed., 2017, 56, 15141 CrossRef CAS PubMed; (h) G. Dilauro, M. Dell’Aera, P. Vitale, V. Capriati and F. M. Perna, Angew. Chem., Int. Ed., 2017, 56, 10200 CrossRef CAS PubMed; (i) G. Dilauro, A. F. Quivelli, P. Vitale, V. Capriati and F. M. Perna, Angew. Chem., Int. Ed., 2019, 58, 1799 CrossRef CAS PubMed.
- (a) A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70 RSC; (b) M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074 CrossRef CAS PubMed; (c) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060 CrossRef CAS PubMed; (d) D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org. Chem., 2016, 612 CrossRef CAS; (e) F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27 CrossRef.
- (a) C. Vidal, J. García-Álvarez, A. Hernán-Gómez, A. R. Kennedy and E. Hevia, Angew. Chem., Int. Ed., 2014, 53, 5969 CrossRef CAS PubMed; (b) C. Vidal, J. García-Álvarez, A. Hernán-Gómez, A. R. Kennedy and E. Hevia, Angew. Chem., Int. Ed., 2016, 55, 16145 CrossRef CAS PubMed; (c) M. J. Rodríguez-Álvarez, J. García-Álvarez, M. Uzelac, M. Fairley, C. T. O'Hara and E. Hevia, Chem. – Eur. J., 2018, 24, 1720 CrossRef PubMed.
- A. Sánchez-Condado, G. A. Carriedo, A. Presa Soto, M. J. Rodríguez-Álvarez, J. García-Álvarez and E. Hevia, ChemSusChem, 2019, 12, 3134 CrossRef PubMed.
- (a) V. Mallardo, R. Rizzi, F. C. Sassone, R. Mansueto, F. M. Perna, A. Salomone and V. Capriati, Chem. Commun., 2014, 50, 8655 RSC; (b) F. C. Sassone, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Commun., 2015, 51, 9459 RSC; (c) C. Prandi, S. Ghinato, G. Dilauro, F. M. Perna, V. Capriati and M. Blangetti, Chem. Commun., 2019, 55, 7741 RSC.
- Y. Hayashi, Chem. Sci., 2016, 7, 866 RSC.
- L. Cicco, M. J. Rodríguez-Álvarez, F. M. Perna, J. García-Álvarez and V. Capriati, Green Chem., 2017, 19, 3069 RSC.
- (a) N. Ríos-Lombardía, C. Vidal, M. Cocina, F. Morís, J. García-Álvarez and J. González-Sabín, Chem. Commun., 2015, 51, 10937 RSC; (b) N. Ríos-Lombardía, C. Vidal, E. Liardo, F. Morís, J. García-Álvarez and J. González-Sabín, Angew. Chem., Int. Ed., 2016, 55, 8691 CrossRef PubMed; (c) M. J. Rodríguez-Álvarez, N. Ríos-Lombardía, S. Schumacher, D. Pérez-Iglesias, F. Morís, V. Cadierno, J. García-Álvarez and J. González-Sabín, ACS Catal., 2017, 7, 7753 CrossRef; (d) E. Liardo, R. González-Fernández, N. Ríos-Lombardía, F. Morís, J. García-Álvarez, V. Cadierno, P. Crochet, F. Rebolledo and J. González-Sabín, ChemCatChem, 2018, 10, 4676 CrossRef CAS; (e) L. Cicco, N. Ríos-Lombardía, M. J. Rodríguez-Álvarez, F. Morís, F. M. Perna, V. Capriati, J. García-Álvarez and J. González-Sabín, Green Chem., 2018, 20, 3468 RSC.
- (a) E. Liardo, N. Ríos-Lombardía, F. Morís, F. Rebolledo and J. González-Sabín, ACS Catal., 2017, 7, 4768 CrossRef CAS; (b) E. Liardo, N. Ríos-Lombardía, F. Morís, J. González-Sabín and F. Rebolledo, Eur. J. Org. Chem., 2018, 3031 CrossRef CAS; (c) L. Marx, N. Ríos-Lombardía, P. Süss, M. Höhne, F. Morís, J. González-Sabín and P. Berglund, Eur. J. Org. Chem., 2020, 510 CrossRef CAS.
- (a) H. Reich, Chem. Rev., 2013, 113, 7130 CrossRef CAS PubMed; (b) N. D. Bartolo, J. A. Read, E. M. Valentín and K. A. Woerpel, Chem. Rev., 2020, 120, 1513 CrossRef CAS PubMed.
- (a) S. Yamazaki and S. Yamabe, J. Org. Chem., 2002, 67, 9346 CrossRef CAS PubMed; (b) M. Hatano, T. Matsumura and K. Ishihara, Org. Lett., 2005, 7, 573 CrossRef CAS PubMed; (c) M. Hatano, S. Suzuki and K. Ishihara, J. Am. Chem. Soc., 2006, 128, 9998 CrossRef CAS PubMed.
- We have observed experimentally that the addition of higher amounts of n-BuLi under these conditions did not increase the final yield of the desired alcohol 5a.
- G. Osztrovszky, T. Holm and R. Madsen, Org. Biomol. Chem., 2010, 8, 3402 RSC . However, Capriati et al. have reported that several Grignard reagents can be successfully added to carbonyl derivatives (similarly to organolithiums reagents) under “on water” conditions (see ref. 8d).
- (a) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed; (b) R. M. Butler and A. G. Coyne, J. Org. Chem., 2015, 80, 1809 CrossRef CAS PubMed; (c) R. N. Buttler and A. G. Coyle, Org. Biomol. Chem., 2016, 14, 9945 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc03768k |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2020 |