Themed collection 1st International Conference on Noncovalent Interactions

1st International Conference on Noncovalent Interactions
Welcome to this themed collection of RSC articles entitled: 1st International Conference on Noncovalent Interactions.

New J. Chem., 2019,43, 13312-13314
https://doi.org/10.1039/C9NJ90113B
Organic crystal engineering beyond the Pauling hydrogen bond
The application of weaker intermolecular attractions to the formation of crystals with desired physical and chemical properties is described. Representative structural examples are analysed critically and approaches are outlined for the future development of this important area.
CrystEngComm, 2015,17, 7448-7460
https://doi.org/10.1039/C5CE01069A
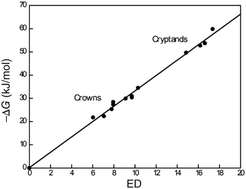
Quantification of noncovalent interactions – promises and problems
Quantification of noncovalent interactions is the key for the understanding of binding mechanisms, of biological systems, for the design of drugs, their delivery and for the design of receptors for separations, sensors, actuators, or smart materials.
New J. Chem., 2019,43, 15498-15512
https://doi.org/10.1039/C9NJ03325D
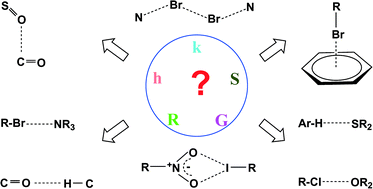
Hydrogen bonds, and σ-hole and π-hole bonds – mechanisms protecting doublet and octet electron structures
For various interactions electron charge shifts try to protect the former doublet or octet electronic structure of the Lewis acid centre.
Phys. Chem. Chem. Phys., 2017,19, 29742-29759
https://doi.org/10.1039/C7CP06393H
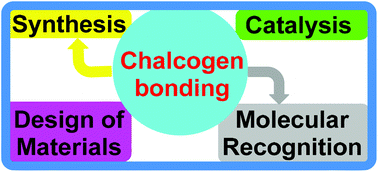
Chalcogen bonding in synthesis, catalysis and design of materials
This Perspective highlights the use of chalcogen bonding as a versatile synthon for the synthesis, catalysis and construction of both organic and inorganic materials.
Dalton Trans., 2017,46, 10121-10138
https://doi.org/10.1039/C7DT01685A
Harnessing non-covalent interactions to exert control over regioselectivity and site-selectivity in catalytic reactions
This perspective examines the progress that has been made in using non-covalent interactions to control regioselectivity and site-selectivity in catalysis.

Chem. Sci., 2017,8, 864-877
https://doi.org/10.1039/C6SC04157D
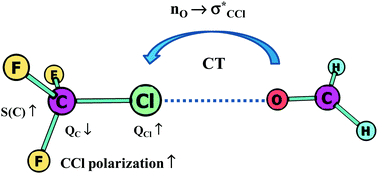
Hydrogen bonds with fluorine. Studies in solution, in gas phase and by computations, conflicting conclusions from crystallographic analyses
Hydrogen bonds with organic fluorine and other halogens as acceptors show a consistent picture in gas and solution phase in agreement with computations, whereas conflicting conclusions were drawn from crystallographic analyses.
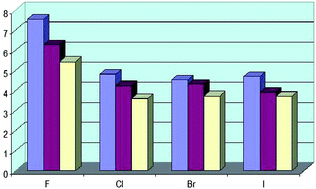
Chem. Sci., 2012,3, 1381-1394
https://doi.org/10.1039/C2SC00764A
Non-covalent interactions in biomacromolecules
Biomolecular building blocks (H-bonded and stacked base pairs) determine the structure of biomolecules (DNA).

Phys. Chem. Chem. Phys., 2007,9, 5291-5303
https://doi.org/10.1039/B704781A
Co-crystal synthesis: fact, fancy, and great expectations
Some strategies for driving co-crystal synthesis using a variety of competing non-covalent interactions are presented.
Chem. Commun., 2018,54, 14047-14060
https://doi.org/10.1039/C8CC08135B
Far infrared spectroscopy of hydrogen bonding collective motions in complex molecular systems
Far infrared spectroscopy as a tool for the study of inter and intramolecular interactions in complex molecular structures.
Chem. Commun., 2017,53, 8389-8399
https://doi.org/10.1039/C7CC03496B
Halogen bonding anion recognition
The development of solution-based anion receptor molecules which exploit halogen bonding interactions is an emerging area of research. This Feature Article reviews recent advances which have been made in this rapidly developing field, surveying the use of iodoperfluoroarene, haloimidazolium and halotriazole/triazolium halogen-bond-donor motifs in anion receptor design and describing the application of mechanically interlocked rotaxane and catenane frameworks as halogen bonding anion host systems.
Chem. Commun., 2016,52, 8645-8658
https://doi.org/10.1039/C6CC03638D
Polyaromatic N-heterocyclic carbene ligands and π-stacking. Catalytic consequences
This article highlights how π-stacking interactions have an important influence on the catalytic properties of transition metal complexes decorated with rigid polyaromatic ligands.
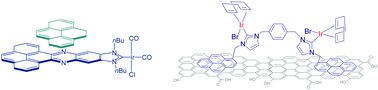
Chem. Commun., 2016,52, 5777-5787
https://doi.org/10.1039/C6CC02017H
Hydrogen bonding networks of nalidixic acid–copper(II) complexes
The formation of hydrogen bonding networks of nalidixic acid–Cu(II) complexes is discussed and may be a possible pathway leading to improved properties and increased efficiency of this antibiotic.
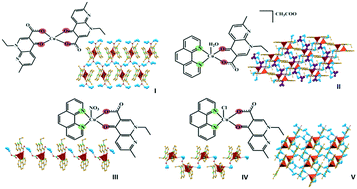
CrystEngComm, 2019,21, 7199-7203
https://doi.org/10.1039/C9CE01057B
Noncovalent interactions in the design of bis-azo dyes
A perfluorinated aromatic link was used as a synthon in the design of bis-azo dyes.
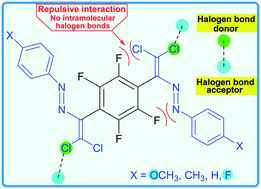
CrystEngComm, 2019,21, 5032-5038
https://doi.org/10.1039/C9CE00956F
[2+2] Halogen-bonded boxes employing azobenzenes
Herein, we report the synthesis and crystal structures of three [2+2] supramolecular boxes assembled by halogen bonding.
![Graphical abstract: [2+2] Halogen-bonded boxes employing azobenzenes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9CC03061A&imageInfo.ImageIdentifier.Year=2019)
Chem. Commun., 2019,55, 8768-8771
https://doi.org/10.1039/C9CC03061A
“C–H⋯π Interaction” regulates the stereoselectivity in olefin polymerization
DFT calculations reveal that a coordinating THF plays an essential role in regulating the stereoselectivity via C–H⋯π noncovalent interaction.
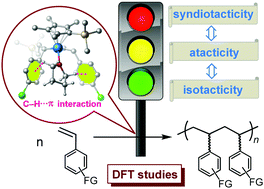
Chem. Commun., 2019,55, 6689-6692
https://doi.org/10.1039/C9CC02165E
Chirality-dependent halogen bonds in axially chiral quinazolin-4-one derivatives bearing ortho-halophenyl groups
In the crystals of racemic axially chiral quinazolinones, the formation of intermolecular halogen bonds was detected.
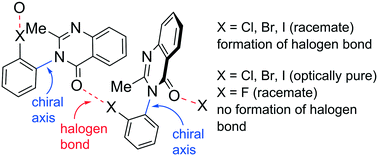
CrystEngComm, 2019,21, 3385-3389
https://doi.org/10.1039/C9CE00320G
Halogen bonding of the aldehyde oxygen atom in cocrystals of aromatic aldehydes and 1,4-diiodotetrafluorobenzene
Novel halogen bonded cocrystals of aromatic aldehydes have been synthesized. We present the halogen bond acceptor potential of the aldehyde group oxygen atom in competition with the hydroxy, methoxy and pyridine groups.
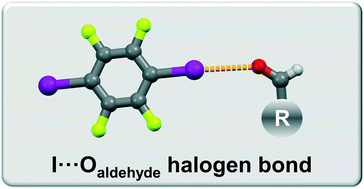
CrystEngComm, 2019,21, 3251-3255
https://doi.org/10.1039/C9CE00340A
Light-driven control of the composition of a supramolecular network
All-photonic and reversible switching of the composition of a supramolecular network is enabled by employing a dithienylethene guest.
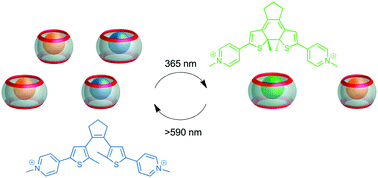
Chem. Commun., 2019,55, 4335-4338
https://doi.org/10.1039/C9CC00922A
The diiodomethyl-sulfonyl moiety: an unexplored halogen bond-donor motif
The α-iodosulfone moiety acts as a new and effective halogen bond donor system in the solid state and in solution.
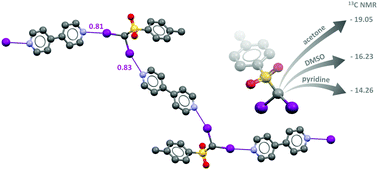
Chem. Commun., 2019,55, 4234-4237
https://doi.org/10.1039/C9CC01092K
Hexagonal array formation by intermolecular halogen bonding using a binary blend of linear building blocks: STM study
A bicomponent blend of linear building blocks leads to intermolecular halogen bonding, resulting in the formation of hexagonal arrays.
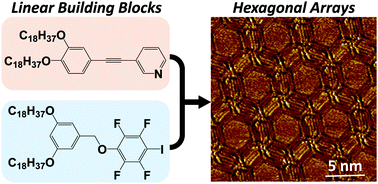
Chem. Commun., 2019,55, 3955-3958
https://doi.org/10.1039/C9CC00532C
Halogen bonding in UiO-66 frameworks promotes superior chemical warfare agent simulant degradation
Herein, a series of halogenated UiO-66 derivatives was synthesized and analyzed for the breakdown of the CWA simulant dimethyl-4-nitrophenyl phosphate (DMNP) to analyze ligand effects.
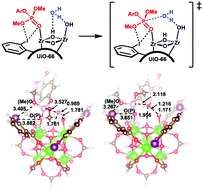
Chem. Commun., 2019,55, 3481-3484
https://doi.org/10.1039/C9CC00642G
Controlled α-mono- and α,α-di-halogenation of alkyl sulfones using reagent–solvent halogen bonding
The direct and selective α-halogenation of alkyl sulfones was achieved via base-mediated electrophilic halogenation, where reagent–solvent halogen bonding was found to control the selectivity through alteration of the effective size of the halogen source.
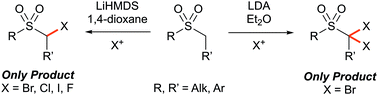
Chem. Commun., 2019,55, 2912-2915
https://doi.org/10.1039/C9CC00550A
Halogen bonding effects on the outcome of reactions at metal centres
Key findings regarding the effects of ligand preorganisation via halogen bonding on the outcome of reactions at rhodium are reported.

Chem. Commun., 2019,55, 2380-2383
https://doi.org/10.1039/C8CC08884E
Aryl-platform-based tetrapodal 2-iodo-imidazolium as an excellent halogen bond receptor in aqueous medium
The graphic shows a halogen bonding interaction between a tetrapodal platform attached to a 2-iodo-imidazole unit and bromide in water.
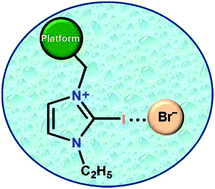
Chem. Commun., 2019,55, 1506-1509
https://doi.org/10.1039/C8CC09937E
Ratiometric DNA sensing with a host–guest FRET pair
A host–guest FRET pair based on a carboxyfluorescein-labelled cucurbit[7]uril and DAPI was developed to sense DNA ratiometrically.
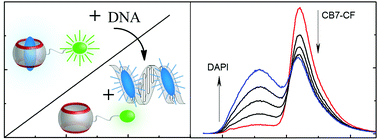
Chem. Commun., 2019,55, 671-674
https://doi.org/10.1039/C8CC09126A
The nature of interactions of benzene with CF3I and CF3CH2I
Weak though structure determining interactions exist between benzene and F3CI or F3CCH2I; their natures are quite different and lead to different types of networks.
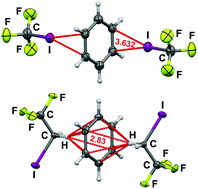
Chem. Commun., 2019,55, 175-178
https://doi.org/10.1039/C8CC08980A
Correlating thermochromic and mechanochromic phosphorescence with polymorphs of a complex gold(I) double salt with infinite aurophilicity
Something learnt from a golden trio: polymorphs of a [Au(NHC)2][Au(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)2] double salt allow an understanding of the thermochromic and mechanochromic phosphorescence of the gold(I) complexes with extended aurophilicity.
N)2] double salt allow an understanding of the thermochromic and mechanochromic phosphorescence of the gold(I) complexes with extended aurophilicity.
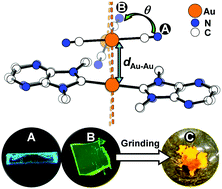
Chem. Commun., 2018,54, 12844-12847
https://doi.org/10.1039/C8CC05210G
Assisted π-stacking: a strong synergy between weak interactions
An exceptionally strong synergy between aromatic π-stacking and n → π* interaction.
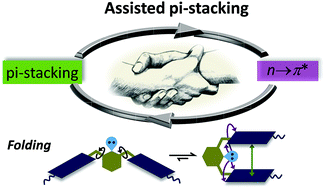
Chem. Commun., 2018,54, 12186-12189
https://doi.org/10.1039/C8CC07207H
A rare example of a phosphine as a halogen bond acceptor
The cocrystal of triphenylphosphine with 1,3,5-trifluoro-2,4,6-triiodobenzene features a rare, moderately strong, and linear phosphorus–iodine halogen bond.
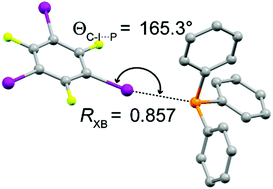
Chem. Commun., 2018,54, 11041-11043
https://doi.org/10.1039/C8CC06019C
Hydrogen bonding versus π-interactions: their key competition in sildenafil solvates
Herein we report the X-ray characterization of four sildenafil solvates where the conformation of the pyrazolo[3,4-d]pyrimidine and phenyl rings depends on the solvent.
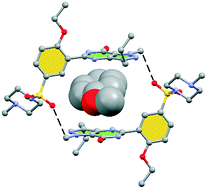
CrystEngComm, 2018,20, 4526-4530
https://doi.org/10.1039/C8CE00567B
Continuum of covalent to intermolecular bonding in the halogen-bonded complexes of 1,4-diazabicyclo[2.2.2]octane with bromine-containing electrophiles
The Br⋯N bonds in a series of halogen-bonded complexes change gradually from the typical intermolecular to the traditional covalent bond.
![Graphical abstract: Continuum of covalent to intermolecular bonding in the halogen-bonded complexes of 1,4-diazabicyclo[2.2.2]octane with bromine-containing electrophiles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC04629H&imageInfo.ImageIdentifier.Year=2018)
Chem. Commun., 2018,54, 8060-8063
https://doi.org/10.1039/C8CC04629H
Characterization of the short O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯O
C⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π-hole tetrel bond in the solid state
C π-hole tetrel bond in the solid state
An in-depth structure database investigation and experimental charge density analysis of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯O
C⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π-hole tetrel bonds.
C π-hole tetrel bonds.
![Graphical abstract: Characterization of the short O [[double bond, length as m-dash]] C⋯O [[double bond, length as m-dash]] C π-hole tetrel bond in the solid state](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CE00697K&imageInfo.ImageIdentifier.Year=2018)
CrystEngComm, 2018,20, 3308-3312
https://doi.org/10.1039/C8CE00697K
Palladium(II) N-heterocyclic allenylidene complexes with extended intercationic Pd⋯Pd interactions and MMLCT phosphorescence
Pallas's shine: extended intercationic Pd⋯Pd contacts of 3.30 Å show distinct MMLCT transitions and low-energy emissions.
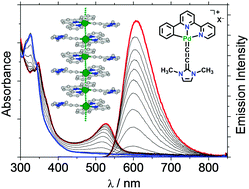
Chem. Commun., 2018,54, 5319-5322
https://doi.org/10.1039/C8CC01652F
Model molecules to classify CH⋯O hydrogen-bonds
A set of molecules locked in the CH⋯O H-bonding conformation has been used to correlate the magnitude of the downfield shift of the 1H NMR signal due to the bridging hydrogen with the hybridization state of the acceptor oxygen and the CH⋯O H-bond strength.
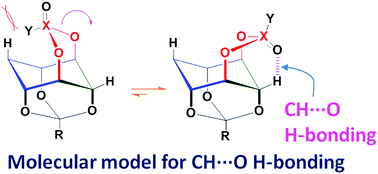
Chem. Commun., 2018,54, 4629-4632
https://doi.org/10.1039/C8CC01653D
Diamondoid architectures from halogen-bonded halides
Halide ions and tetraiodoethynyl-featured tetraphenylmethane are successfully assembled into robust diamond-like networks in the presence of tetraphenylphosphonium cations.
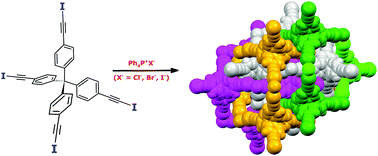
Chem. Commun., 2018,54, 607-610
https://doi.org/10.1039/C7CC08839F
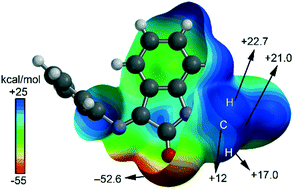
Structure guided or structure guiding? Mixed carbon/hydrogen bonding in a bis-Schiff base of N-allyl isatin
A supramolecular motif listed as ‘carbon bonded’ or ‘hydrogen bonded’ may have the character of both. We highlight the hybrid character of the non-covalent interaction in a bis-Schiff base of N-allyl isatin by combining theory and experiment.
CrystEngComm, 2018,20, 150-154
https://doi.org/10.1039/C7CE01697B
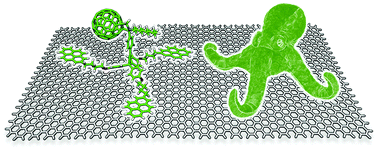
Non-covalent graphene nanobuds from mono- and tripodal binding motifs
Dispersion forces govern the interaction of graphene with mono- and tripodal pyrene–[60]fullerene derivatives and direct the formation of graphene nanobuds.
Chem. Commun., 2017,53, 12402-12405
https://doi.org/10.1039/C7CC07836F
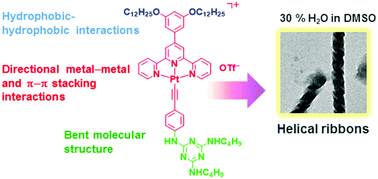
A rational molecular design of triazine-containing alkynylplatinum(II) terpyridine complexes and the formation of helical ribbons via Pt⋯Pt, π–π stacking and hydrophobic–hydrophobic interactions
The self-assembly of strategically designed triazine-containing alkynylplatinum(II) terpyridine complexes yielded sophisticated helical ribbons through a balance of multiple non-covalent interactions.
Chem. Commun., 2017,53, 11349-11352
https://doi.org/10.1039/C7CC06293A
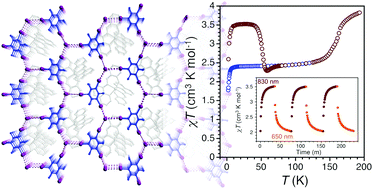
Photoinduced reversible spin-state switching of an FeIII complex assisted by a halogen-bonded supramolecular network
The organization of a molecular FeIII complex embedded in a halogen-bonded 2D network is chemically tuned to trigger temperature- and light-induced spin-state switching.
Chem. Commun., 2017,53, 10283-10286
https://doi.org/10.1039/C7CC03797J
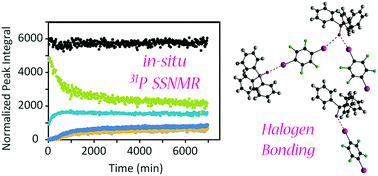
A kinetic study of mechanochemical halogen bond formation by in situ31P solid-state NMR spectroscopy
In situ 31P solid-state NMR studies of mechanochemical halogen bond formation provide insights into the cocrystallisation process and an estimate of the activation energy.
Chem. Commun., 2017,53, 9930-9933
https://doi.org/10.1039/C7CC05051H
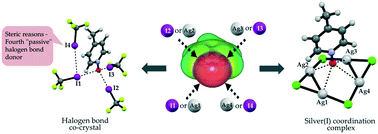
Tridentate C–I⋯O−–N+ halogen bonds
N-Oxides can act as tridentate halogen bond acceptors, or as tetradentate ligands in a pseudo-μ4 mode with silver(I).
CrystEngComm, 2017,19, 4960-4963
https://doi.org/10.1039/C7CE01381G
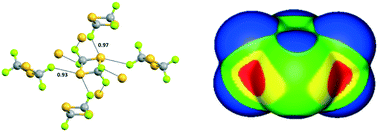
Fluorination promotes chalcogen bonding in crystalline solids
Flurorine promotes the electrophilicity of sulfur to the point that chalcogen bond formation affects the crystal packing in the solid.
CrystEngComm, 2017,19, 4955-4959
https://doi.org/10.1039/C7CE01070B
Coordinated nitrate anions can be directional π-hole donors in the solid state: a CSD study
Within the CSD sp2 O-atoms cluster closer to the π-hole of NO3− when nitrate is coordinated to a metal.
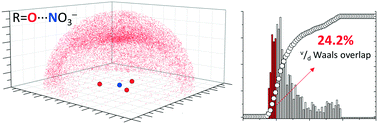
CrystEngComm, 2017,19, 4485-4488
https://doi.org/10.1039/C7CE01266G
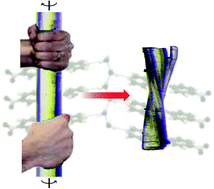
A hand-twisted helical crystal based solely on hydrogen bonding
Third-generation crystal engineering: using halogen bond/hydrogen bond equivalence.
Chem. Commun., 2017,53, 6371-6374
https://doi.org/10.1039/C7CC02970E
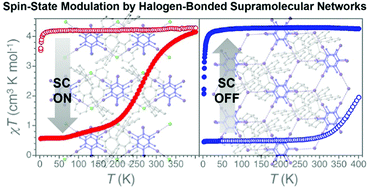
Spin-state modulation of molecular FeIII complexes via inclusion in halogen-bonded supramolecular networks
The cationic complex [Fe(qsal)2]+ (Hqsal = N-(8-quinolyl)salicylaldimine) is encapsulated in halogen-bonded 1D and 2D supramolecular networks to modulate its spin-state.
Chem. Commun., 2017,53, 4989-4992
https://doi.org/10.1039/C7CC01943B
Ethynyl hydrogen bonds and iodoethynyl halogen bonds: a case of synthon mimicry
The common electrostatic features of ethynyl and iodoethynyl hydrogen- and halogen-bond donors, respectively, lead to synthon mimicry which can be employed in synthetic crystal engineering for the construction of identical supramolecular assemblies in the solid-state.

CrystEngComm, 2017,19, 11-13
https://doi.org/10.1039/C6CE02201D
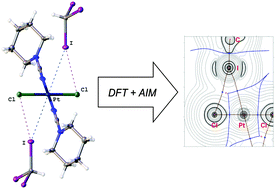
Halogen bonding between metal centers and halocarbons
Metal-involving halogen bonding was detected in a series of associates of CHI3 with trans-[PtX2(NCNAlk2)2] (X = Cl, Br).
Chem. Commun., 2016,52, 5565-5568
https://doi.org/10.1039/C6CC01107A
Topology analysis reveals supramolecular organisation of 96 large complex ions into one geometrical object
Demonstrating the power of network topology analysis on a 46 000 Å3 unit cell containing 12 independent Ag(I) coordination entities.
CrystEngComm, 2016,18, 1883-1886
https://doi.org/10.1039/C5CE02490K
H-bond competition experiments in solution and the solid state
When two different H-bond acceptors compete for a single H-bond donor, the outcomes in crystal structures of simple molecules are consistent with the results of solution phase measurements in non-polar solvents.
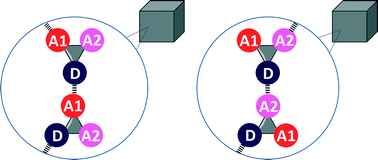
CrystEngComm, 2016,18, 394-397
https://doi.org/10.1039/C5CE02223A
The non-planarity of the benzene molecule in the X-ray structure of the chelated bismuth(III) heteroboroxine complex is not supported by quantum mechanical calculations
The competition of two σ-hole(Bi)⋯π interactions is responsible for disorder of the benzene moiety in the crystal of bismuth(III) heteroboroxine–benzene complex.
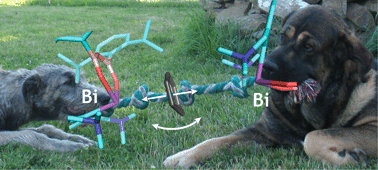
Dalton Trans., 2016,45, 462-465
https://doi.org/10.1039/C5DT04381F
Unexpected synthesis of an Au2In2 tetrametallatricyclic complex from α-aminophosphines and formation of Au–In–P and Ag–In–P nanomaterials
Four Au–(μ-phosphinite)–In units form an unprecedented Au2In2 12-membered metallacycle which intersects at the In centres an 8-membered ring containing two In–μ-phosphinate linkages, resulting in a tricyclic structure.
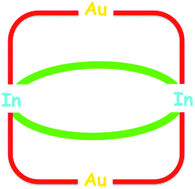
Dalton Trans., 2015,44, 16242-16246
https://doi.org/10.1039/C5DT02457A
Porous organic hydrate crystals: structure and dynamic behaviour of water clusters
Infinite water clusters with a T5(2) motif were observed in porous crystals of 4-nitrostyrylpyridine hydrochloride, the behavior of which was revealed by solid-state 17O NMR spectroscopic analyses.
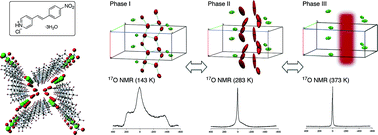
CrystEngComm, 2015,17, 5629-5633
https://doi.org/10.1039/C4CE02517B
The N-atom in [N(PR3)2]+ cations (R = Ph, Me) can act as electron donor for (pseudo) anti-electrostatic interactions
A CSD analysis and DFT study reveal that the nitrogen lone-pair in [N(PPh3)2]+ is partially intact and involved in intramolecular hydrogen bonding.
![Graphical abstract: The N-atom in [N(PR3)2]+ cations (R = Ph, Me) can act as electron donor for (pseudo) anti-electrostatic interactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5CE00352K&imageInfo.ImageIdentifier.Year=2015)
CrystEngComm, 2015,17, 3768-3771
https://doi.org/10.1039/C5CE00352K
Anion–π interactions and positive electrostatic potentials of N-heterocycles arise from the positions of the nuclei, not changes in the π-electron distribution
The positive ESPs that underlie anion-binding by N-heterocycles do not stem from a depletion of π-electron density, as widely assumed.
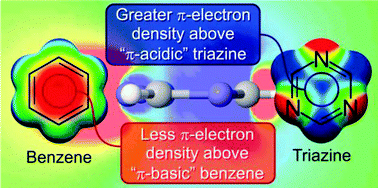
Chem. Commun., 2014,50, 11118-11121
https://doi.org/10.1039/C4CC05304D
Photoresponsive liquid crystals based on halogen bonding of azopyridines
A series of photoresponsive halogen-bonded liquid crystals (LCs) were successfully constructed using molecular halogen and azopyridine compounds, which show interesting properties of photoinduced phase transition upon UV irradiation.
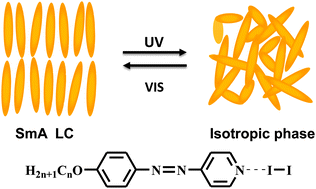
Chem. Commun., 2014,50, 9647-9649
https://doi.org/10.1039/C4CC02344G
Weak becomes strong: remarkable strength of C–H⋯π hydrogen bond in the presence of O–H⋯O hydrogen bonds in the crystal stabilization
We report crystallographic evidence for the significance of C–H⋯π hydrogen bonds in the crystal stabilization of 1,4-di-O-benzoyl-myo-inositol. The strength of this otherwise weak hydrogen bond matches with the strength of O–H⋯O hydrogen bonds.
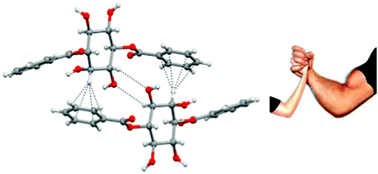
CrystEngComm, 2013,15, 1676-1679
https://doi.org/10.1039/C3CE26894B
Interactions between dehydrobenzo[12]annulene (DBA) and gas molecules: do the preorganized acetylenes work cooperatively?
Intermolecular interactions of the cyclic conjugated molecule (DBA) with hydrogen, nitrogen and carbon dioxide molecules were evaluated by high level ab initio calculations.
![Graphical abstract: Interactions between dehydrobenzo[12]annulene (DBA) and gas molecules: do the preorganized acetylenes work cooperatively?](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CP43091F&imageInfo.ImageIdentifier.Year=2012)
Phys. Chem. Chem. Phys., 2012,14, 13918-13921
https://doi.org/10.1039/C2CP43091F
Halogen bond effect on bundling of hydrogen bonded 2-fold helical columns
Bundling of the hydrogen bonded 2-fold helical columns has been altered on the basis of a robust halogen bond between an iodine and anionic oxygen atom. This alteration causes a change of the space groups of the resulting crystals.

CrystEngComm, 2012,14, 5749-5752
https://doi.org/10.1039/C2CE26081F
Hydrogen bond assisted activation of a dinitrile towards nucleophilic attack
The possibility of regioselective activation of a dinitrile towards nucleophilic attack using a resonance-assisted hydrogen bond system is demonstrated.

Chem. Commun., 2011,47, 7248-7250
https://doi.org/10.1039/C1CC11696G
Concerted halogen and hydrogen bonding in [RuI2(H2dcbpy)(CO)2]⋯I2⋯(CH3OH)⋯I2⋯[RuI2(H2dcbpy)(CO)2]
A new type of concerted halogen bond–hydrogen bond interaction was found in the solid state structure of [RuI2(H2dcbpy)(CO)2]⋯I2⋯(MeOH)⋯I2⋯[RuI2(H2dcbpy)(CO)2].
![Graphical abstract: Concerted halogen and hydrogen bonding in [RuI2(H2dcbpy)(CO)2]⋯I2⋯(CH3OH)⋯I2⋯[RuI2(H2dcbpy)(CO)2]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C0CC05726F&imageInfo.ImageIdentifier.Year=2011)
Chem. Commun., 2011,47, 3427-3429
https://doi.org/10.1039/C0CC05726F
Halogenated building blocks for 2D crystal engineering on solid surfaces: lessons from hydrogen bonding
We test whether the similarities between halogen and hydrogen bonds could be used to design a surface-confined halogen-bond based network.
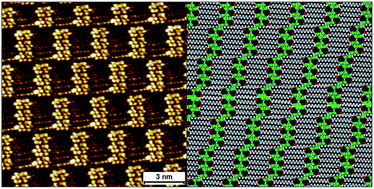
Chem. Sci., 2019,10, 3881-3891
https://doi.org/10.1039/C8SC04499F
Site-selective nitrenoid insertions utilizing postfunctionalized bifunctional rhodium(II) catalysts
Site-selective nitrenoid insertions are made possible with a postfunctionalized dirhodium(II)-catalyst equipped with a remote hydrogen bonding site.

Chem. Sci., 2019,10, 3324-3329
https://doi.org/10.1039/C8SC05733H
Supramolecular cage encapsulation as a versatile tool for the experimental quantification of aromatic stacking interactions
A Double Mutant Cycle is built up using a supramolecular cage that binds two aromatic carboxylates in a stacked geometry is used to quantify aromatic stacking interactions.
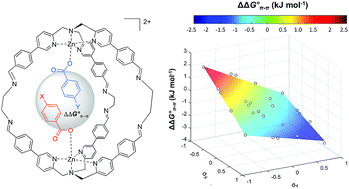
Chem. Sci., 2019,10, 1466-1471
https://doi.org/10.1039/C8SC04406F
Nanobowls with controlled openings and interior holes driven by the synergy of hydrogen bonding and π–π interaction
Nanobowls with controlled openings and interior holes are created by self-assembly of homopolymers with hydrogen bonding and π–π interaction.
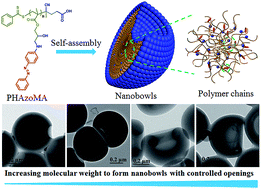
Chem. Sci., 2019,10, 657-664
https://doi.org/10.1039/C8SC03995J
Enantioselective fluorination of homoallylic alcohols enabled by the tuning of non-covalent interactions
Multivariate correlation analysis, including designer π-interaction derived parameters, was applied to the study of the enantioselective fluorination of homoallylic alcohols via chiral anion phase transfer (CAPT) catalysis.
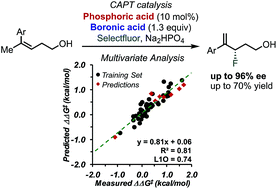
Chem. Sci., 2018,9, 7153-7158
https://doi.org/10.1039/C8SC02223B
Self-complementary nickel halides enable multifaceted comparisons of intermolecular halogen bonds: fluoride ligands vs. other halides
Studies of X–Ni–C6F4I⋯X–Ni–C6F4I halogen-bonded networks reveal pronounced differences between fluoride (X = F) and other halides: the 19F-MAS NMR spectrum is a sensitive probe of the halogen bond.
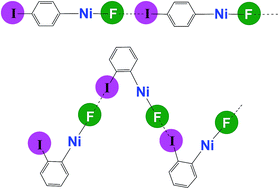
Chem. Sci., 2018,9, 3767-3781
https://doi.org/10.1039/C8SC00890F
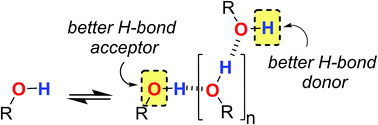
Polarisation effects on the solvation properties of alcohols
Alcohol solvents are significantly more polar than expected based on the measured H-bonding properties of monomeric alcohols in dilute solution.
Chem. Sci., 2018,9, 88-99
https://doi.org/10.1039/C7SC04890D
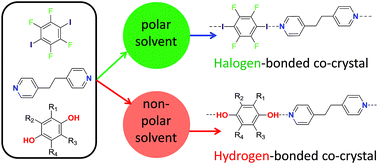
Hydrogen bonding vs. halogen bonding: the solvent decides
Choice of solvent is used to direct the formation of either hydrogen bonds or halogen bonds in competitive self-assembly.
Chem. Sci., 2017,8, 5392-5398
https://doi.org/10.1039/C7SC01801K
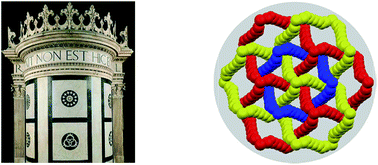
Halogen bonded Borromean networks by design: topology invariance and metric tuning in a library of multi-component systems
Borromean rings in black and white marble decorate Cappella Ruccellai (left, Florence) and were self-assembled under halogen bond control (right).
Chem. Sci., 2017,8, 1801-1810
https://doi.org/10.1039/C6SC04478F
About this collection
Noncovalent interactions (hydrogen, noble gases, halogen, chalcogen, pnictogen, tetrel and triel bonds, as well as cation-π, anion-π, lone pair-π, π-π stacking, agostic, pseudo-agostic, anagostic, dispersion-driven, lipophilic, etc.) concern weak forces of attraction formed between different molecules (intermolecular) or fragments of the same molecule (intramolecular). While these weak interactions were firstly taken into consideration by van der Waals in 1873, the understanding of their crucial role in synthesis, catalysis, crystal engineering, pharmaceutical design, molecular biology, molecular recognition, materials, etc. has been increasingly explored in the last few decades. Thus, it is timely to establish a general/regular series of International Conferences on Noncovalent Interactions (ICNI), the first of which is to be held on 2-6 September 2019 in Lisbon. The conference aims to bring together scientists from around the world working on this field in order to exchange ideas, discuss recent advances and future directions/plans.
Articles in this web themed collection will be added below as soon as possible after they are published. Please return to this page frequently to see the collection growth.