Hydrogen bonds, and σ-hole and π-hole bonds – mechanisms protecting doublet and octet electron structures
Abstract
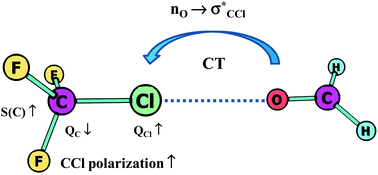
The hydrogen bond interaction and σ-hole and π-hole bonds are steered by the same mechanisms. There is electron charge transfer from the Lewis base to the Lewis acid unit, and further, for various interactions the same mechanisms try to protect the former electronic structure of the Lewis acid centre. The increase of the polarization of bonds to this centre seems to be the common effect. In the case of the A–H⋯B hydrogen bond it is the increase of the polarization of the A–H bond connected with the outflow of the electron charge from the H-atom to the A-centre. For other interactions the outflow of electron charge from the Lewis acid centre is also observed. These electron charge shifts try to protect the doublet/octet structure of the acidic centre. The extremely strong interaction is often equivalent to the formation of new covalent bonds or it may lead to chemical reactions. Numerous interactions may be treated as the preliminary stages of chemical reactions: hydrogen bond – proton transfer, dihydrogen bond – molecular hydrogen release, tetrel bond – SN2 reaction, etc.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...