Organic crystal engineering beyond the Pauling hydrogen bond
Abstract
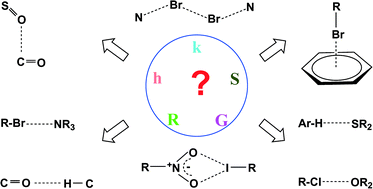
The hydrogen bond, as described by Pauling, involves a hydrogen atom bonded to a strongly electronegative element interacting with an electron lone pair of a second electronegative atom. This molecular interaction is used extensively in crystal engineering since it is commonplace, well understood, strong, and highly directional. There are, of course, many further types of intermolecular attractions that operate within organic crystals. This article provides an overview of some of these alternatives. Examples such as halogen bonding and aromatic interactions are also well known and popular. Others, however, have received scant attention for crystal engineering purposes. This tutorial account provides an introduction to the role of these weaker forces in producing new crystals with predicted physical and chemical properties. Diverse examples of their crystal behaviour are illustrated. This relatively undeveloped area of research offers considerable potential for future crystal engineering applications.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions

 Please wait while we load your content...
Please wait while we load your content...