Abstract
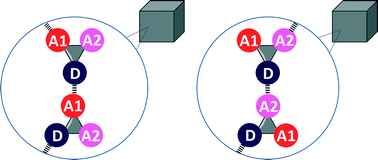
The H-bonding outcomes in crystal structures of simple molecules, where two potential H-bonds can be formed, have been used to calculate relative H-bond probabilities for 59 combinations of H-bond donors and H-bond acceptors. H-bond probabilities are shown to correlate well with the difference in solution phase free energy between the two competing H-bonds.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions

 Please wait while we load your content...
Please wait while we load your content...