Hydrogen bonding versus π-interactions: their key competition in sildenafil solvates†
Abstract
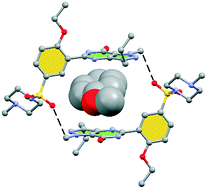
Herein we report the X-ray characterization of four sildenafil solvates where the conformation of the pyrazolo[3,4-d]pyrimidine and phenyl rings depends on the solvent. It conditions the formation of an apparently innocent intramolecular H-bond that has a remarkable influence on the solid state architecture of the sildenafil solvates. DFT calculations indicate that a delicate balance between the energies of H-bonding and π–π (or lp–π) interactions are crucial.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...