Enantioselective fluorination of homoallylic alcohols enabled by the tuning of non-covalent interactions†
Abstract
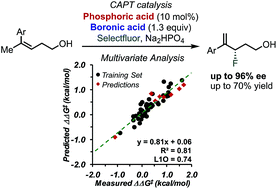
The study of the enantioselective fluorination of homoallylic alcohols via chiral anion phase transfer (CAPT) catalysis using an in situ generated directing group is described. Multivariate correlation analysis, including designer π-interaction derived parameters, revealed key structural features affecting the selectivity at the transition state (TS). Interpretation of the parameters found in the model equation highlights the key differences as well as similarities for the reaction of homoallylic and allylic substrates. A similar T-shaped π-interaction was found to occur between the substrate and the catalyst. The tuning of this crucial interaction by identification of the best combination of phosphoric acid catalyst and boronic acid directing group allowed for the development of a methodology to access γ-fluoroalkenols in typically high enantioselectivities (up to 96% ee).
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...