Themed collection Editors’ collection: Catalytic Organic Transformations

Combining transition metals and transient directing groups for C–H functionalizations
This review is about the momentous evolution of transient directing group assisted C–H functionalizations promoted by three major second row transition metals.
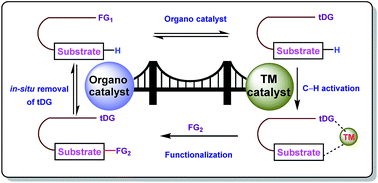
RSC Adv., 2018,8, 19456-19464
https://doi.org/10.1039/C8RA03230K
Recent metal-catalysed approaches for the synthesis of cyclopenta[b]indoles
This review provides a summary of recent metal-catalysed approaches for the synthesis of cyclopenta[b]indoles.
![Graphical abstract: Recent metal-catalysed approaches for the synthesis of cyclopenta[b]indoles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8RA03480J&imageInfo.ImageIdentifier.Year=2018)
RSC Adv., 2018,8, 18576-18588
https://doi.org/10.1039/C8RA03480J
Palladium-catalyzed carbonylative transformation of aryl chlorides and aryl tosylates
The developments in the carbonylative transformations of aryl chlorides and aryl tosylates have been collected and discussed.
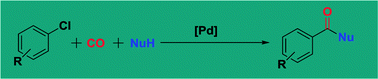
RSC Adv., 2016,6, 83831-83837
https://doi.org/10.1039/C6RA18388C
Amine synthesis via transition metal homogeneous catalysed hydrosilylation
This review summarizes the preparation of amines involving homogeneous transition metal catalysed hydrosilylation including reductions of imines, amides, nitro and nitriles, reductive aminations and N-methylation of amines with CO2.

RSC Adv., 2016,6, 57603-57625
https://doi.org/10.1039/C6RA10494K
Atom-economic dehydrogenative amide synthesis via ruthenium catalysis
Recent developments of ruthenium-catalyzed atom-economic transformations for dehydrogenative amide synthesis are reviewed.
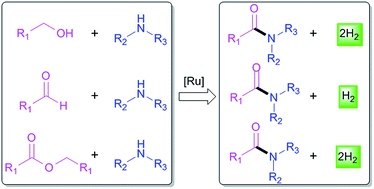
RSC Adv., 2016,6, 55599-55607
https://doi.org/10.1039/C6RA10643A
Ruthenium-catalyzed direct arylations with aryl chlorides
Aryl chlorides are readily available at lower cost than the corresponding bromides and iodides, but are much more challenging as substrates in metal-catalyzed cross-couplings.
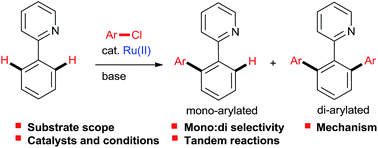
RSC Adv., 2016,6, 30875-30885
https://doi.org/10.1039/C6RA02742C
Asymmetric Mannich reaction: highly enantioselective synthesis of 3-amino-oxindoles via chiral squaramide based H-bond donor catalysis
A facile formation of 3-aminooxindole derivatives from the reaction of 1,3-diketones with isatin (N-Boc) via an asymmetric Mannich reaction catalyzed by chiral cinchona alkaloid based squaramide containing H-bond donor catalysts.

RSC Adv., 2016,6, 84242-84247
https://doi.org/10.1039/C6RA16877A
B(C6F5)3 catalysed 1,6-conjugate allylation of para-quinone methides: expedient access to allyl diarylmethanes
An efficient protocol for the synthesis of unsymmetrical allyl diarylmethanes through a Lewis acid catalysed 1,6-conjugate addition of allylsilanes to para-quinone methides is described.
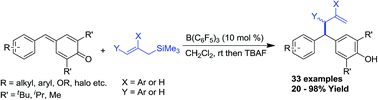
RSC Adv., 2016,6, 80718-80722
https://doi.org/10.1039/C6RA19069C
Organocatalytic one-pot asymmetric synthesis of functionalized spiropyrazolones via a Michael-aldol sequential reaction
An efficient organocatalytic method was developed for synthesizing functionalized spiropyrazolone derivatives by using a Michael-aldol consecutive reaction.
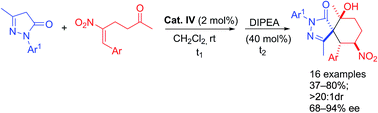
RSC Adv., 2016,6, 77474-77480
https://doi.org/10.1039/C6RA13923J
Ruthenium-catalyzed 1,2,3-triazole directed intermolecular C–H amidation of arenes with sulfonyl azides
An efficient strategy for the synthesis of 2-(2H-1,2,3-triazole-2-yl)aniline derivatives from 2-aryl-1,2,3-triazoles and sulfonylazides through ruthenium-catalyzed intermolecular C–H amidation is achieved.
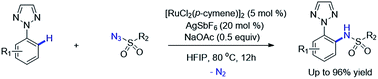
RSC Adv., 2016,6, 68929-68933
https://doi.org/10.1039/C6RA14020C
Palladium-catalyzed Si–C bond-forming silylation of aryl iodides with hydrosilanes: an enhanced enantioselective synthesis of silicon-stereogenic silanes by desymmetrization
An enantioselective Pd-catalyzed silicon–carbon bond-forming silylation reaction of aryl iodides with hydrosilanes for the synthesis of silicon-stereogenic silanes has been developed with good enantioselectivity under mild conditions.
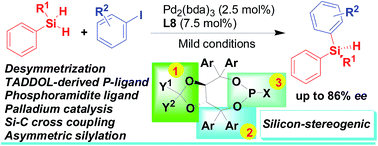
RSC Adv., 2016,6, 67113-67117
https://doi.org/10.1039/C6RA12873D
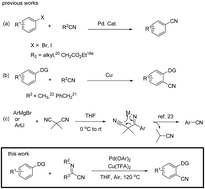
α-Iminonitrile: a new cyanating agent for the palladium catalyzed C–H cyanation of arenes
An efficient palladium-catalyzed C–H cyanation reaction of arenes using α-iminonitrile as a new cyanating reagent has been developed.
RSC Adv., 2016,6, 64234-64238
https://doi.org/10.1039/C6RA14512D
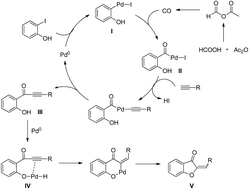
Selective palladium-catalyzed carbonylative synthesis of aurones with formic acid as the CO source
A general and practical strategy has been developed to prepare aurone derivatives.
RSC Adv., 2016,6, 62810-62813
https://doi.org/10.1039/C6RA13615J
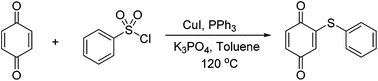
Copper and triphenylphosphine-promoted sulfenylation of quinones with arylsulfonyl chlorides
The copper and triphenylphosphine-promoted sulfenylation of quinones with arylsulfonyl chlorides has been developed under mild conditions with moderate to good yields. This is the first time to prepare arylthioquinones with arylsulfonyl chlorides.
RSC Adv., 2016,6, 62298-62301
https://doi.org/10.1039/C6RA11301J
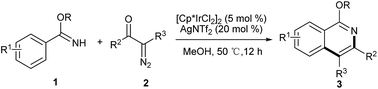
Ir(III)-catalyzed synthesis of isoquinolines from benzimidates and α-diazocarbonyl compounds
We report herein a tandem Ir(III)-catalyzed C–H activation and annulation reactions for the synthesis of isoquinolines. This novel method affords an alternative strategy for the construction of diverse and useful isoquinoline derivatives.
RSC Adv., 2016,6, 57371-57374
https://doi.org/10.1039/C6RA10045G
Magnesium iodide-catalyzed synthesis of 2-substituted quinazolines using molecular oxygen and visible light
We disclose a novel and efficient synthesis of 2-substituted quinazolines by aerobic photooxidative reaction catalyzed by magnesium iodide.
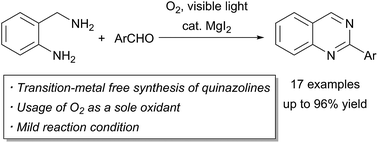
RSC Adv., 2016,6, 56892-56895
https://doi.org/10.1039/C6RA04073J
Gold-catalyzed formation of indole derivatives from 2-alkynyl arylazides and oxygen-containing heterocycles
Gold-catalyzed ring-opening reactions of oxygen-containing heterocycles.
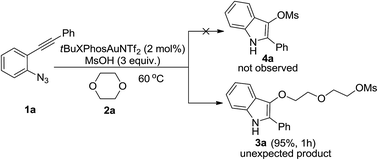
RSC Adv., 2016,6, 56319-56322
https://doi.org/10.1039/C6RA09761H
Cu(I)-catalyzed double C–H amination: synthesis of 2-iodo-imidazo[1,2-a]pyridines
An iodine/CuI mediated double oxidative C–H amination reaction has been developed for the synthesis of 2-iodo-imidazo[1,2-a]pyridines, which can serve as active pharmaceutical ingredients (API) of marketed drugs like saripidem and nicopidem.
![Graphical abstract: Cu(i)-catalyzed double C–H amination: synthesis of 2-iodo-imidazo[1,2-a]pyridines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA02953A&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 38033-38036
https://doi.org/10.1039/C6RA02953A
Copper(II)-catalyzed remote sulfonylation of aminoquinolines with sodium sulfinates via radical coupling
Many various aminoquinolines-derived sulfones were obtained in moderate to high yields via copper(II)-catalyzed direct C(sp2)–H sulfonylation of aminoquinolines with sodium sulfinates.
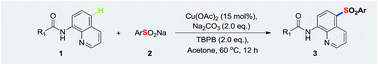
RSC Adv., 2016,6, 37173-37179
https://doi.org/10.1039/C6RA04013F
Copper-catalyzed N-formylation of amines with CO2 under ambient conditions
N-Formylation of amines with CO2 and PhSiH3 catalyzed by a copper complex.
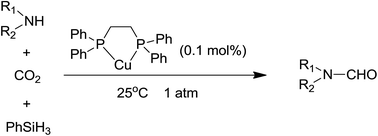
RSC Adv., 2016,6, 32370-32373
https://doi.org/10.1039/C6RA05199E
A calcium catalysed regioselective (5-exo dig) tandem process for the synthesis of fully substituted furans
An efficient and highly regioselective synthesis of fully substituted furans has been described using Ca(OTf)2. The reaction proceeds through tandem alkylation, 5-exo dig cyclisation and isomerization under solvent free conditions.
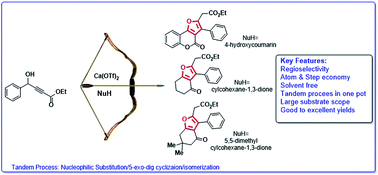
RSC Adv., 2016,6, 28865-28870
https://doi.org/10.1039/C6RA03042D
A facile and efficient method for the synthesis of alkynone by carbonylative Sonogashira coupling using CHCl3 as the CO source
A facile and efficient method for the synthesis of alkynones by a Pd-catalyzed carbonylative Sonogashira coupling reaction starting from aryl iodide, terminal alkyne and chloroform (CHCl3) as the CO source is described.
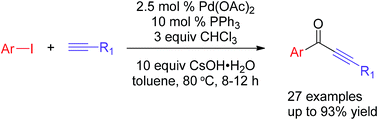
RSC Adv., 2016,6, 28442-28446
https://doi.org/10.1039/C6RA02424F
Cobalt(III)-catalyzed synthesis of pyrroles from enamides and alkynes
An efficient and regioselective cobalt-catalyzed synthesis of multi-substituted pyrroles is reported by the use of readily available enamides and alkynes.
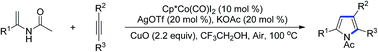
RSC Adv., 2016,6, 24768-24772
https://doi.org/10.1039/C6RA01992G
Copper-catalyzed and iodide-promoted aerobic C–C bond cleavage/C–N bond formation toward the synthesis of amides
A copper-catalyzed and iodide promoted aerobic C–C bond cleavage/C–N bond formation reaction between ketone and simple amine was developed toward the synthesis of amides.

RSC Adv., 2016,6, 24349-24352
https://doi.org/10.1039/C6RA02153K
Cobalt(II)-catalysed transfer hydrogenation of olefins
Catalytic transfer hydrogenation of olefins is achieved by an earth-abundant metal cobalt catalyst. A range of alkene substrates including those with functional groups have been hydrogenated in high yields.
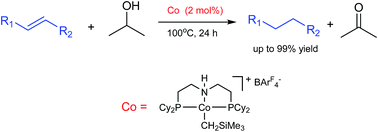
RSC Adv., 2016,6, 22419-22423
https://doi.org/10.1039/C6RA02021F
Highly diastereo-/enantioselective Cu-catalyzed propargylic alkylations of propargyl acetates with cyclic enamines
Highly diastereo- and enantioselective copper-catalyzed propargylic alkylations of morpholine-derived cyclic enamines have been developed by the employment of a less sterically hindered chiral tridentate P,N,N-ligand.
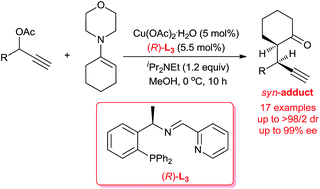
RSC Adv., 2016,6, 14763-14767
https://doi.org/10.1039/C5RA25627E
Palladium-catalyzed N-arylsulfonamide formation from arylsulfonyl hydrazides and nitroarenes
Various N-arylsulfonamides were prepared from nitroarenes and arylsulfonyl hydrazides via hydrogen transfer methodology.
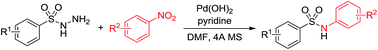
RSC Adv., 2016,6, 13010-13013
https://doi.org/10.1039/C5RA26588F
An efficient synthesis of sulfonated quinoline diones by copper catalyzed sulfonylation of activated alkenes with sulfonylhydrazides
A novel and highly efficient cascade synthesis of sulfonated quinoline dione derivatives from N-(2-cyanoaryl) methylacrylamides and sulfonylhydrazides in good yields is described.
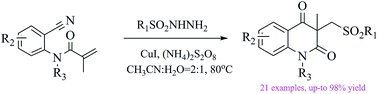
RSC Adv., 2016,6, 11754-11757
https://doi.org/10.1039/C5RA27878C
Copper-catalyzed cycloaddition between hydrogen phosphonates and activated alkenes: synthesis of phosphonoisoquinolinediones
A new and general method for the synthesis of phosphonoisoquinolinediones has been achieved through copper-catalyzed phosphonation–cyclization of various methacryloylbenzamides with P(O)H compounds.
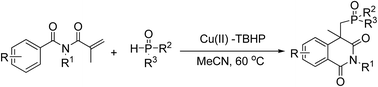
RSC Adv., 2016,6, 303-306
https://doi.org/10.1039/C5RA22570A
Ni(II)-catalyzed mono-selective ortho-arylation of unactivated aryl C–H bonds utilizing amino acids as a directing group
The nickel(II)-catalyzed ortho-arylation of unactivated C–H bonds utilizing amino acids as directing groups with aryl iodides or bromides as coupling electrophiles is described.
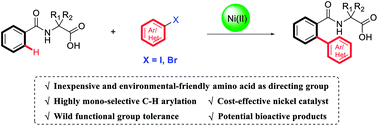
RSC Adv., 2019,9, 10820-10824
https://doi.org/10.1039/C9RA00749K
Synthesis of N-aryl β-amino acid derivatives via Cu(II)-catalyzed asymmetric 1,4-reduction in air
Non-precious metal copper-catalyzed asymmetric 1,4-hydrosilylation of β-aryl or β-alkyl-substituted N-aryl β-enamino esters proceeded in air in high yields and excellent enantioselectivities (26 examples, 90–98% ee).
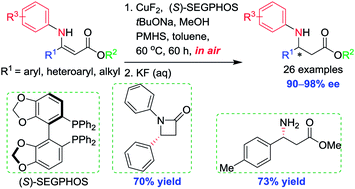
RSC Adv., 2019,9, 9187-9192
https://doi.org/10.1039/C9RA01203F
Cross coupling of benzylammonium salts with boronic acids using a well-defined N-heterocyclic carbene–palladium(II) precatalyst
N-heterocyclic carbene–palladium(II)-catalyzed cross-coupling of benzylammonium salts with arylboronic acids for the synthesis of diarylmethane derivatives via C–N bond activation has been developed.

RSC Adv., 2019,9, 5738-5741
https://doi.org/10.1039/C8RA10439E
I2-catalyzed intramolecular oxidative amination of C(sp3)–H bond: efficient access to 3-acylimidazo[1,2-a]pyridines under neat condition
An efficient and green protocol for the synthesis of 3-acylimidazo[1,2-a]pyridines utilizing I2 as a catalyst and H2O2 as an oxidant under neat condition or in water was developed.
![Graphical abstract: I2-catalyzed intramolecular oxidative amination of C(sp3)–H bond: efficient access to 3-acylimidazo[1,2-a]pyridines under neat condition](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8RA10118C&imageInfo.ImageIdentifier.Year=2019)
RSC Adv., 2019,9, 2381-2385
https://doi.org/10.1039/C8RA10118C
Chan–Lam coupling reaction of sulfamoyl azides with arylboronic acids for synthesis of unsymmetrical N-arylsulfamides
An efficient method was developed for the synthesis of unsymmetrical N-arylsulfamides using sulfamoyl azides and arylboronic acids in the presence of 10 mol% of copper chloride as the catalyst.
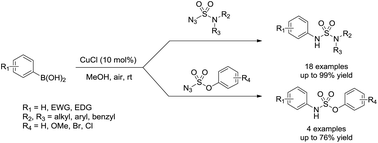
RSC Adv., 2019,9, 2493-2497
https://doi.org/10.1039/C8RA09219B
One-pot synthesis of chiral alcohols from alkynes by CF3SO3H/ruthenium tandem catalysis
A simple and compatible catalytic system for one-pot transformation of alkynes into chiral alcohols was developed.
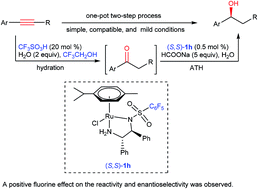
RSC Adv., 2018,8, 14829-14832
https://doi.org/10.1039/C8RA02224K
Palladium-catalyzed intramolecular C–H arylation of 2-halo-N-Boc-N-arylbenzamides for the synthesis of N–H phenanthridinones
Pd(t-Bu3P)2/KOAc proved to be a good combination for one-pot synthesis of N–H phenanthridinones with up to 95% yield.

RSC Adv., 2018,8, 13879-13890
https://doi.org/10.1039/C8RA02099J
Cobalt-catalyzed C(sp3)–H/C(sp2)–H oxidative coupling between alkanes and benzamides
A direct cobalt-catalyzed oxidative coupling between C(sp2)–H in unactivated benzamides and C(sp3)–H in simple alkanes, ethers and toluene derivatives was explored.
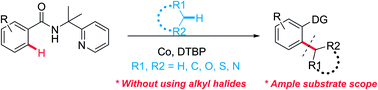
RSC Adv., 2018,8, 13454-13458
https://doi.org/10.1039/C8RA01377B
Cobalt(II)-catalyzed remote C5-selective C–H sulfonylation of quinolines via insertion of sulfur dioxide
A cobalt(II)-catalyzed reaction for highly selective C5-sulfonylation of quinolines via insertion of sulfur dioxide is developed, leading to diverse sulfonated quinolines in moderate to good yields.

RSC Adv., 2017,7, 51313-51317
https://doi.org/10.1039/C7RA11363C
Asymmetric synthesis of chromene skeletons via organocatalytic domino reactions of in situ generated ortho-quinone methide with malononitrile and β-functionalized ketone
Enantioselective organocatalytic domino reactions of in situ generated ortho-quinone methides with malononitrile and β-functionalized ketones have been developed. This strategy could generate various chiral chromenes in high yields and stereoselectivities.
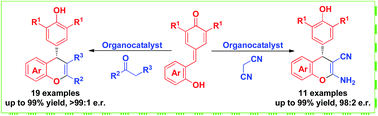
RSC Adv., 2017,7, 39216-39220
https://doi.org/10.1039/C7RA08157J
CuCl/air-mediated oxidative coupling reaction of imidazoheterocycles with N-aryl glycine esters
A novel CuCl/air-mediated oxidative cross-coupling of imidazoheterocycles with glycine esters has been developed.

RSC Adv., 2017,7, 30152-30159
https://doi.org/10.1039/C7RA05303G
Rh(III)-catalyzed sequential C–H activation and annulation: access to N-fused heterocycles from arylazoles and α-diazocarbonyl compounds
Valuable N-fused heterocycles were successfully obtained via Rh(III)-catalyzed sequential C–H activation and annulation.
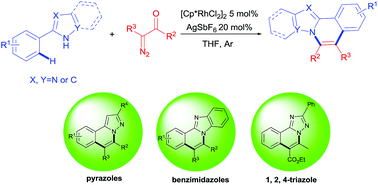
RSC Adv., 2017,7, 20548-20552
https://doi.org/10.1039/C7RA03262E
Palladium catalyzed Suzuki cross-coupling of benzyltrimethylammonium salts via C–N bond cleavage
A Pd catalyzed Suzuki cross-coupling of a benzyltrimethylammonium salt is described. This reaction offers a highly efficient approach to diarylmethanes and also paves the way for the application of benzyltrimethylammonium salts in Pd catalyzed cross-coupling reactions.
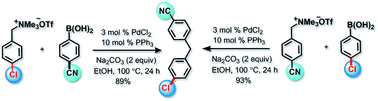
RSC Adv., 2017,7, 15805-15808
https://doi.org/10.1039/C7RA02549A
Eosin Y-catalyzed photooxidation of triarylphosphines under visible light irradiation and aerobic conditions
A novel method for Eosin Y-catalyzed photooxidation of triarylphosphines under visible light irradiation and aerobic conditions was reported.
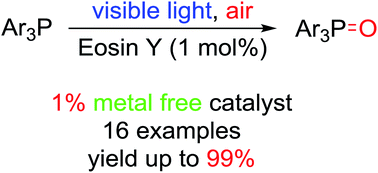
RSC Adv., 2017,7, 13240-13243
https://doi.org/10.1039/C6RA25469A
Oxidative bicyclization of N-tethered 1,7-enynes toward polycyclic 3,4-dihydroquinolin-2(1H)-ones via site-selective decarboxylative C(sp3)–H functionalization
A new Ag-catalyzed oxidative bicyclization of N-tethered 1,7-enynes with alkylcarboxylic acids for forming 41 examples of polycyclic 3,4-dihydroquinolin-2(1H)-ones has been established using readily accessible K2S2O8 as an oxidant.
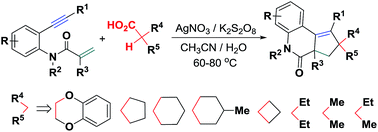
RSC Adv., 2017,7, 9693-9703
https://doi.org/10.1039/C6RA28589A
Visible light-mediated decarboxylative amination of indoline-2-carboxylic acids catalyzed by Rose Bengal
A visible-light-induced decarboxylative amination of N-protected indoline-2-carboxylic acids and azodicarboxylate esters catalyzed by Rose Bengal has been developed.
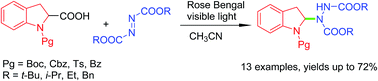
RSC Adv., 2016,6, 96693-96699
https://doi.org/10.1039/C6RA17524D
Copper-catalyzed rapid C–H nitration of 8-aminoquinolines by using sodium nitrite as the nitro source under mild conditions
We report the first example of copper(II) catalyzed remote C–H nitration of 8-aminoquinoline amides by using sodium nitrite as nitration reagent under mild conditions in 1 minute which undergoes single electron process.
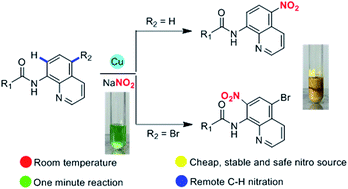
RSC Adv., 2016,6, 89979-89983
https://doi.org/10.1039/C6RA19583K
Aqueous biphasic iron-catalyzed asymmetric transfer hydrogenation of aromatic ketones
For the first time, an iron(II) catalyst is used in the biphasic asymmetric transfer hydrogenation (ATH) of ketones to enantioenriched alcohols employing water and potassium formate as the proton and hydride source, respectively.
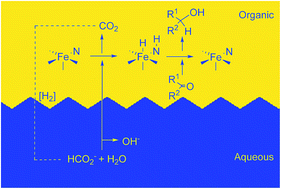
RSC Adv., 2016,6, 88580-88587
https://doi.org/10.1039/C6RA22538A
Iron nitrate/TEMPO-catalyzed aerobic oxidative synthesis of quinazolinones from alcohols and 2-aminobenzamides with air as the oxidant
A highly efficient, one-pot Fe(NO3)3/TEMPO-catalyzed protocol for aerobic oxidative synthesis of quinazolinones from easily accessible primary alcohols and 2-aminobenzamides with molecular oxygen as the terminal oxidant.
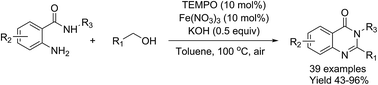
RSC Adv., 2016,6, 65196-65204
https://doi.org/10.1039/C6RA12164K
Cu(II)-catalyzed C6-selective C–H thiolation of 2-pyridones using air as the oxidant
Cu(II)-catalyzed C6-selective C–H thiolation of 2-pyridones with aryl and alkyl disulfides to provide functional thioethers has been achieved.

RSC Adv., 2016,6, 57441-57445
https://doi.org/10.1039/C6RA04388G
L-Isoleucine derived bifunctional phosphine catalyses asymmetric [3 + 2]-annulation of allenyl-esters and -ketones with ketimines
L-Isoleucine derived bifunctional N-acylaminophosphine catalyzed a [3 + 2]-annulation reaction between allenyl carbonyl compounds and isatinimines to afford a facile and asymmetric access to 3,2′-dihydropyrrolyl spirooxindoles.
![Graphical abstract: l-Isoleucine derived bifunctional phosphine catalyses asymmetric [3 + 2]-annulation of allenyl-esters and -ketones with ketimines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA12387B&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 56537-56543
https://doi.org/10.1039/C6RA12387B
Palladium-catalyzed radical cascade difluoroalkylation/cyclization of acrylamide with ethyl difluorobromoacetate
An efficient Pd(0)-catalyzed radical cascade difluoroalkylation/cyclization of acrylamide with ethyl difluorobromoacetate was described.
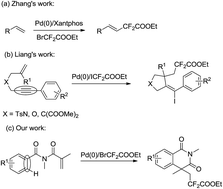
RSC Adv., 2016,6, 51703-51709
https://doi.org/10.1039/C6RA05744F
Rh(III)-catalyzed C–H oxidative ortho-olefination of arenes using 7-azaindole as a directing group and utilization in the construction of new tetracyclic heterocycles containing a 7-azaindole skeleton
A wide range of Rh(III)-catalyzed ortho-olefinated 7-azaindole derivatives as well as novel tetracyclic heterorings were achieved, which could served as useful starting materials for the construction of biological molecules.
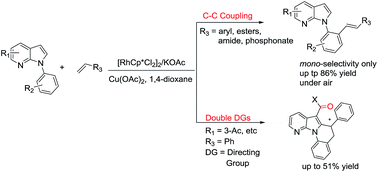
RSC Adv., 2016,6, 48205-48211
https://doi.org/10.1039/C6RA07478B
Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions with structurally diverse nucleophiles: scope and limitations
The asymmetric palladium-catalysed alkylation of structurally diverse nucleophiles, including alcohols, activated methylene compounds, indoles, and aromatic amines, led to the formation of corresponding products in good yields and high enantioselectivities (up to 99% ee).
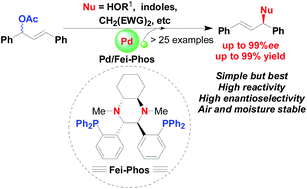
RSC Adv., 2016,6, 45495-45502
https://doi.org/10.1039/C6RA09657C
Highly efficient synthesis of primary amides via aldoximes rearrangement in water under air atmosphere catalyzed by an ionic ruthenium pincer complex
An ionic Ru pincer complex was demonstrated to be highly efficient catalyst for conversion of aldoximes and aldehydes into corresponding primary amides with environmental benignity, operational convenience and catalytic loading as low as 0.5 mol%.
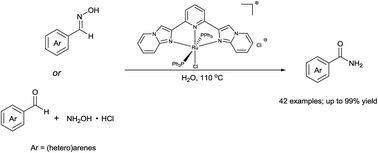
RSC Adv., 2016,6, 37093-37098
https://doi.org/10.1039/C6RA07515K
Copper-catalyzed aerobic oxidative decarboxylative amination of arylacetic acids: a facile access to 2-arylquinazolines
An efficient copper-catalyzed oxidative decarboxylative amination of arylacetic acids with 2-aminobenzoketones and ammonium acetate under an oxygen atmosphere was first developed.
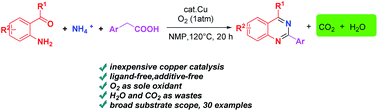
RSC Adv., 2016,6, 36192-36197
https://doi.org/10.1039/C6RA04195G
Co(acac)2/O2-catalyzed oxidative isocyanide insertion with 2-vinylanilines: efficient synthesis of 2-aminoquinolines
A series of 2-aminoquinolines were afforded by Co(acac)2 catalyzed isocyanide insertion with 2-vinylanilines under an O2 atmosphere.
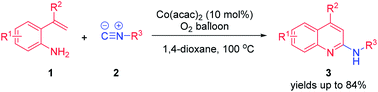
RSC Adv., 2016,6, 32467-32470
https://doi.org/10.1039/C6RA03216H
Ligand-free palladium-catalyzed facile construction of tetra cyclic dibenzo[b,h][1,6]naphthyridine derivatives: domino sequence of intramolecular C–H bond arylation and oxidation reactions
A ligand-free Pd-catalyzed approach has been developed for the synthesis of dibenzo-fused naphthyridines.
![Graphical abstract: Ligand-free palladium-catalyzed facile construction of tetra cyclic dibenzo[b,h][1,6]naphthyridine derivatives: domino sequence of intramolecular C–H bond arylation and oxidation reactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA00505E&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 26993-26999
https://doi.org/10.1039/C6RA00505E
Palladium-catalyzed C-3 desulfitative arylation of indolizines with sodium arylsulfinates and arylsulfonyl hydrazides
Derivatized indolizines were efficiently prepared by direct C-3 arylation of indolizines using sodium arylsulfinates and arylsulfonyl hydrazides in good yields.
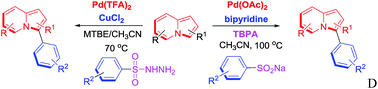
RSC Adv., 2016,6, 21814-21821
https://doi.org/10.1039/C5RA25504J
TBAI/TBHP-catalyzed [3 + 2]cycloaddition/oxidation/aromatization cascade and online ESI-MS mechanistic studies: synthesis of pyrrolo[2,1-a]isoquinolines and indolizino[8,7-b]indoles
A facile [3 + 2]cycloaddition/oxidation/aromatization cascade reaction for the synthesis of pyrrolo[2,1-a]isoquinolines and indolizino[8,7-b]indoles has been developed by employing tert-butyl hydroperoxide (TBHP) and tetrabutylammonium iodide (TBAI).
![Graphical abstract: TBAI/TBHP-catalyzed [3 + 2]cycloaddition/oxidation/aromatization cascade and online ESI-MS mechanistic studies: synthesis of pyrrolo[2,1-a]isoquinolines and indolizino[8,7-b]indoles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5RA24629F&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 2671-2677
https://doi.org/10.1039/C5RA24629F
Rapid threefold cross-couplings with sterically bulky triarylbismuths under Pd–Cu dual catalysis
The threefold cross-coupling reactivity of sterically highly demanding bulky triarylbismuths was addressed with task specific Pd–Cu dual catalytic conditions.
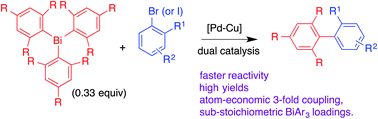
RSC Adv., 2016,6, 1012-1017
https://doi.org/10.1039/C5RA23311A
About this collection
This collection is guest-edited by Associate Editor Professor Thierry Ollevier (Université Laval) and contains selected Reviews, Communications and full Papers published from 2016 to 2019. The collection brings together articles to inspire new authors to submit their best work to the journal, and also highlights the great contributions by authors. It contains papers that go far beyond the theme of 'catalysis in synthetic organic chemistry', illustrating the notability, quality and variety of publications in RSC Advances.
These articles are already among the most highly cited research articles in the journal, illustrating their impact. Subject areas include homogeneous catalysis, asymmetric catalysis, heterocyclic and organometallic chemistry, and method development. Finally, emerging areas such as C–H activation, photocatalysis, organocatalysis and Lewis catalysis have a strong synthetic organic chemistry basis and also find a natural home in this RSC Advances collection.