Fei-Phos ligand-controlled asymmetric palladium-catalyzed allylic substitutions with structurally diverse nucleophiles: scope and limitations†
Abstract
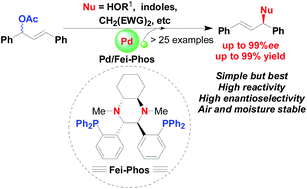
To shed light on the scope and limitations of palladium-catalyzed allylic alkylation in the presence of chiral trans-1,2-diaminocyclohexane-derived Fei-Phos as an effective phosphine ligand, the asymmetric palladium-catalysed alkylation of structurally diverse hard/soft nucleophiles, including allylic etherification of alcohols and the allylic alkylation of activated methylene compounds, indoles, and aromatic amines were investigated in this study; the corresponding products with various functional groups were achieved in good yield and with high enantioselectivity (up to 99% ee).
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...