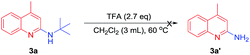Co(acac)2/O2-catalyzed oxidative isocyanide insertion with 2-vinylanilines: efficient synthesis of 2-aminoquinolines†
Pei
Xu
,
Tong-Hao
Zhu
,
Tian-Qi
Wei
,
Shun-Yi
Wang
* and
Shun-Jun
Ji
*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215123, P. R. China. E-mail: shunyi@suda.edu.cn; shunjun@suda.edu.cn; Fax: +86-512-65880307; Tel: +86-512-65880307
First published on 11th March 2016
Abstract
A series of 2-aminoquinolines were afforded by Co(acac)2 catalyzed isocyanide insertion with 2-vinylanilines under an O2 atmosphere. This reaction not only uses low toxicity Co(acac)2 as the catalyst and oxygen as the oxidant, but also allows the construction of new C(sp2)–C(sp2) and C(sp2)–N bonds in a single-step.
2-Aminoquinoline is a type of organic compound, which has good biological and pharmaceutical activities. For instance, the tricycle quipazine has been found to treat depression (Scheme 1).1 The trade name of Aldara is imiquimod (1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine), which has some activity against actinic keratosis and superficial skin cancers.2 VU-WS113 is an anti-tumor drug.3 Thus, a lot of research has been committed to the development of methods to synthesize 2-aminequinolines. There are already some ways to synthesize 2-aminoquinolines: (i) the reducing metal-mediated reaction between nitrophenyl and acrylonitrile under acidic conditions4 and (ii) the transition-metal-catalyzed amination of 2-haloquinolines.5 However, these reactions have some shortcomings, such as harsh reaction conditions and poor functional group tolerance. Therefore, the development of a more highly efficient synthesis of 2-aminoquinoline derivatives is desirable.
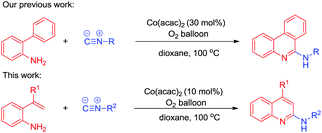
Isocyanides and CO are valuable building blocks and are widely used in the field of organic synthesis.6 Isocyanides play a prominent role in the synthesis of nitrogen-containing heterocycles due to their diversity.7 Recently, there have been a lot of reports on transition metal catalyzed isocyanide insertion reactions.8 For example, our group have reported the synthesis of 6-substituted phenanthridines via the radical reactions of 2-isocyanobiaryls9 and we have successfully developed a cobalt-catalyzed reaction of 2-arylanilines with isocyanides to construct 6-aminophenanthridine derivatives.10 We envisioned that the cobalt-catalyzed insertion reaction strategy might also be applied to promote the reaction of isocyanides with 2-vinylanilines. Herein, we describe the insertion reaction of isocyanides with 2-vinylanilines to construct a series of 2-aminoquinoline derivatives under an O2 atmosphere (Scheme 2).
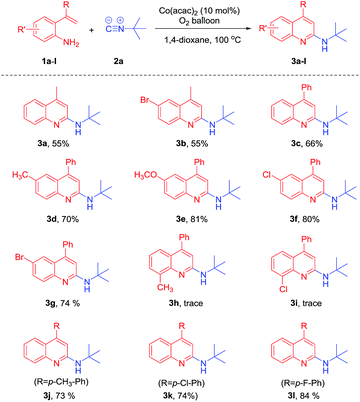
We commenced our studies by treating 2-(prop-1-en-2-yl)aniline 1a with tert-butyl isocyanide 2a in the presence of 20 mol% Co(acac)2 under an oxygen atmosphere at 100 °C. To our delight, N-(tert-butyl)-4-methylquinolin-2-amine 3a was obtained in 50% GC-yield. The structure of 3a was confirmed by IR, NMR and HRMS. With this promising result in hand, we optimized the reaction conditions. No product was detected when the reaction performed without Co(acac)2 (Table 1, entry 2). When the reaction was carried out under an argon atmosphere, only a trace amount of 3a was observed (Table 1, entry 3). Other cobalt salts such as Co(OAc)2·4H2O, Co(NO3)2·6H2O, CoBr2·6H2O, CoSO4·7H2O could not promote the reaction efficiently (Table 1, entries 4–7). Among the solvents tested, 1,4-dioxane was found to be the best solvent choice (Table 1, entries 8–13). The catalyst loading and temperature screening results indicated that 10 mol% Co(acac)2 and 100 °C led to the best result (Table 1, entries 14–18).
| Entry | Catalyst (mol%) | Solvent | Temp. | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.6 mmol), cobalt catalyst, solvent (3 mL), Schlenk tube, O2 balloon, 12 h. b Yields were determined by GC analysis using biphenyl as an internal standard. c The reaction was carried out under an argon atmosphere. d The reaction time was 6 h. e The reaction was carried out for 18 h. | ||||
| 1 | Co(acac)2 (20) | Dioxane | 100 | 50 |
| 2 | — | Dioxane | 100 | 0 |
| 3c | Co(acac)2 (20) | Dioxane | 100 | Trace |
| 4 | Co(OAc)2·4H2O (20) | Dioxane | 100 | 0 |
| 5 | Co(NO3)2·6H2O (20) | Dioxane | 100 | Trace |
| 6 | CoBr2·6H2O (20) | Dioxane | 100 | Trace |
| 7 | CoSO4·7H2O (20) | Dioxane | 100 | 0 |
| 8 | Co(acac)2 (20) | MeCN | Reflux | 27 |
| 9 | Co(acac)2 (20) | Toluene | 100 | 31 |
| 10 | Co(acac)2 (20) | DMSO | 100 | Trace |
| 11 | Co(acac)2 (20) | DMF | 100 | 40 |
| 12 | Co(acac)2 (20) | CHCl3 | Reflux | 18 |
| 13 | Co(acac)2 (20) | THF | Reflux | 35 |
| 14 | Co(acac)2 (5) | Dioxane | 100 | 50 |
| 15 | Co(acac) 2 (10) | Dioxane | 100 | 56 |
| 16 | Co(acac)2 (30) | Dioxane | 100 | 44 |
| 17 | Co(acac)2 (10) | Dioxane | 80 | 43 |
| 18 | Co(acac)2 (10) | Dioxane | 110 | 51 |
| 19d | Co(acac)2 (10) | Dioxane | 100 | 47 |
| 20e | Co(acac)2 (10) | Dioxane | 100 | 54 |
With the optimal conditions in hand, we investigated the substrate scope using various 2-vinyl-anilines. The results are shown in the Table 2. The reaction of 4-bromo-2-(prop-1-en-2-yl)aniline 1b with tert-butyl isocyanide 2a proceeded smoothly to furnish the desired product 3b in 55% yield. The reaction of 2-(1-phenylvinyl)aniline 1c with 2a led to product 3c in 66% yield. 2-(1-Phenylvinyl)aniline derivatives bearing electron-donating groups such as Me and OMe gave 3d and 3e in 70% and 81% yields, respectively. Next, we explored the reaction of 1f and 1g with 2a. It was found that the desired products 3f and 3g could also be obtained in 80% and 74% yield, respectively. However, the reaction of tri-substituted 2-(1-arylvinyl)anilines 1h and 1i only gave trace yields of the desired products 3h and 3i, respectively, due to steric effects. It should be noted that the reaction of other 2-(1-arylvinyl)anilines 1j–l with 2a also proceeded smoothly to give the desired products 3j–l in 73% to 84% yields.
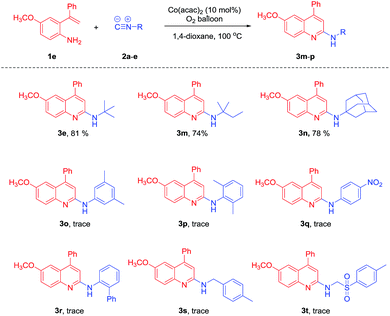
To further study the synthetic utility of our protocol, we investigated the reaction of 1e with different isocyanides under the optimal conditions. As shown in Table 3, the reaction of 2-isocyano-2-methylbutane 2b and 1-isocyanoadamantane 2c proceeded well to afford 2-aminoquinolines 3m and 3n in 74% and 78% yield, respectively. Unfortunately, aryl isocyanides 2o–r failed to afford the desired products. When alkyl isocyanides 1-(isocyanomethyl)-4-methylbenzene and 1-((isocyanomethyl)sulfonyl)-4-methylbenzene were employed, only trace amounts of the desired products were detected. In an attempt to remove the t-Bu group of N-(tert-butyl)-4-methylquinolin-2-amine 3a, we tried the reaction of 3a in the presence of excess trifluoroacetic acid (TFA). Unfortunately, we failed to get 4-methylquinolin-2-amine (Scheme 3).
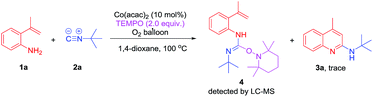
To better understand the mechanism of the reaction, we have explored the cobalt catalysed-reaction of 1a and 2a in the presence of 2.0 equiv. TEMPO (Scheme 4). It was found that only a trace amount of 3a was observed. It should be noted that a TEMPO trapped intermediate 4 was detected by LC-MS, which indicates that the reaction of the aromatic amine with isocyanide might proceed through a radical process.
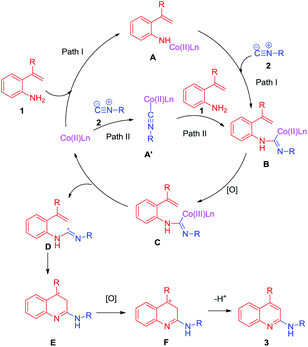
On the basis of relevant literature10,11 and the above experimental results, a plausible mechanism through two possible pathways is proposed in Scheme 5. Initially, 1 reacts with the Co(II) salt to form a cobalt(II) complex A. Then, a cobalt(II) carbene complex B is formed by the reaction of 2 with A (Scheme 4, path I). Another possible pathway involves the reaction of the Co(II) salt with 2 to give another cobalt(II) complex A′. The subsequent reaction of A′ with 1 leads to complex B (Scheme 3, path II). Then, complex B is oxidized to give the intermediate cobalt complex C. Complex C forms radical D and the Co(II) catalyst by homolysis. It is radical D that can be trapped by TEMPO and detected by LC-MS. Otherwise, radical D undergoes tandem intramolecular cyclization and oxidation to form F and finally, the desired 2-aminoquinoline is formed via deprotonation.
Conclusions
In summary, we have demonstrated a novel approach for the cobalt-catalyzed isocyanide insertion reaction of 2-vinylanilines to construct 2-aminoquinolines. The reaction provides a new strategy for constructing 2-aminoquinolines under mild conditions. Further mechanistic studies and application studies are under way in our laboratory.Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (No. 21372174, 21542015), PAPD, and Soochow University for financial support.Notes and references
- (a) A. A. Alhaider, M. A. Abdelkader and E. J. Lien, J. Med. Chem., 1985, 28, 1394 CrossRef CAS; (b) R. Rodriguez and E. Pardo, Psychopharmacologia, 1971, 21, 81 CrossRef; (c) K. Hino, K. Furukawa, Y. Naga and H. Uno, Chem. Pharm. Bull., 1980, 28, 2618 CrossRef CAS.
- K. Turner, Org. Process Res. Dev., 2010, 14, 759 CrossRef CAS.
- C. A. Thorne, A. J. Hanson, J. Schneider, E. Tahinci, D. Orton, C. S. Cselenyi, K. K. Jernigan, K. C. Meyers, B. I. Hang, A. J. Waterson, K. Kim, B. Meloancon, V. P. Ghidu, G. A. Sulikowski, B. L. Fleur, A. Salic, L. A. Lee, D. M. Miller and E. Lee, Nat. Chem. Biol., 2010, 6, 829 CrossRef CAS.
- (a) A. Hamid, A. Elomri and A. Daich, Tetrahedron Lett., 2006, 47, 1777 CrossRef CAS; (b) R. S. Compagnone, A. I. Suarez, J. L. Zambrano, I. C. Pina and J. N. Dominguez, Synth. Commun., 1997, 27, 1631 CrossRef CAS; (c) L. Zhou and Y. Zhang, Synth. Commun., 1998, 28, 3249 CrossRef CAS.
- (a) S.-Z. Ge, R. A. Green and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 1617 CrossRef CAS; (b) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS; (c) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS; (d) Q. Shen and J. F. Hartwig, Org. Lett., 2008, 11, 4109 CrossRef PubMed.
- For recent selected examples, see: (a) T. Vlaar, R. C. Cioc, P. Mampuys, B. U. W. Maes, R. V. A. Orru and E. Ruijter, Angew. Chem., Int. Ed., 2012, 51, 13058 CrossRef CAS; (b) J. V. Geden, A. K. Pancholi and M. Shipman, J. Org. Chem., 2013, 78, 4158 CrossRef CAS; (c) T. Vlaar, R. V. A. Orru, B. U. W. Maes and E. Ruijter, J. Org. Chem., 2013, 78, 10469 CrossRef CAS; (d) T. Vlaar, L. Bensch, J. Kraakman, C. M. L. V. Velde, B. U. W. Maes, R. V. A. Orru and E. Ruijter, Adv. Synth. Catal., 2014, 356, 1205 CrossRef CAS; (e) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS; (f) J. Wang, S. Luo, J. Huang, T. Mao and Q. Zhu, Chem.–Eur. J., 2014, 20, 11220 CrossRef CAS; (g) T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao and S.-J. Ji, Org. Lett., 2015, 17, 1974 CrossRef CAS PubMed; (h) G. C. Senadi, W. P. Hu, S. S. K. Boominathan and J. J. Wang, Chem.–Eur. J., 2015, 21, 998 CrossRef CAS; (i) V. Esté vez, G. V. Baelen, B. H. Lentferink, T. Vlaar, E. Janssen, B. U. W. Maes, R. V. A. Orru and E. Ruijter, ACS Catal., 2014, 4, 40 CrossRef; (j) T.-H. Zhu, T.-Q. Wei, S.-Y. Wang and S.-J. Ji, Org. Chem. Front., 2015, 2, 259 RSC; (k) D. Zheng, S. Li and J. Wu, Org. Lett., 2012, 14, 2655 CrossRef CAS; (l) J. Liu, Z. Liu, N. Wu, P. Liao and X. Bi, Chem.–Eur. J., 2014, 20, 2154 CrossRef CAS; (m) J. Liu, Z. Liu, P. Liao, L. Zhang, T. Tu and X. Bi, Angew. Chem., Int. Ed., 2015, 54, 10618 CrossRef CAS; (n) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505 RSC; (o) A. V. Lygin and A. Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS; (p) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (q) J. Ferguson, F. Zeng and H. Alper, Org. Lett., 2012, 14, 5602 CrossRef CAS; (r) J. Ferguson, F. Zeng, N. Alwis and H. Alper, Org. Lett., 2013, 15, 1998 CrossRef CAS; (s) X.-G. Liu, S.-S. Zhang, C.-Y. Jiang, J.-Q. Wu, Q. Li and H. Wang, Org. Lett., 2015, 17, 5404 CrossRef CAS.
- For reviews, see: (a) I. Ugi, Angew. Chem., Int. Ed., 1962, 1, 8 CrossRef; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef; (d) S. Sadjadi and M. M. Heravi, Tetrahedron, 2011, 67, 2707 CrossRef CAS; (e) H. Jiang, H. Gao, B. Liu and W. Wu, Chem. Commun., 2014, 50, 15348 RSC; (f) Y. Fang, S.-Y. Wang, X.-B. Shen and S.-J. Ji, Org. Chem. Front., 2015, 2, 1338 RSC; (g) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef; (h) X. Wang, S.-Y. Wang and S.-J. Ji, J. Org. Chem., 2014, 79, 8577 CrossRef CAS; (i) X. Wang, X.-P. Xu, S.-Y. Wang, W. Zhou and S.-J. Ji, Org. Lett., 2013, 15, 4246 CrossRef CAS; (j) T. Buyck, Q. Wang and J. Zhu, J. Am. Chem. Soc., 2014, 136, 11524 CrossRef CAS.
- For recent selected examples of a somophilic isocyanide insertion reaction, see: (a) G.-N. Wang, T.-H. Zhu, S.-Y. Wang, T.-Q. Wei and S.-J. Ji, Tetrahedron, 2014, 70, 8079 CrossRef CAS; (b) Y. Kobiki, S. Kawaguchi and A. Ogawa, Org. Lett., 2015, 17, 3490 CrossRef CAS; (c) G. Qiu, G. Liu, S. Pu and J. Wu, Chem. Commun., 2012, 48, 2903 RSC; (d) G. Qiu, Y. He and J. Wu, Chem. Commun., 2012, 48, 3836 RSC; (e) S. Kamijo, T. Jin and Y. Yamamoto, J. Am. Chem. Soc., 2001, 123, 9453 CrossRef CAS; (f) P. Dang, W. Zeng and Y. Liang, Org. Lett., 2015, 17, 34 CrossRef CAS; (g) Q. Gao, P. Zhou, F. Liu, W.-J. Hao, C. Yao, B. Jiang and S.-J. Tu, Chem. Commun., 2015, 51, 9519 RSC; (h) X. Huang, S. Xu, Q. Tan, M. Gao, M. Li and B. Xu, Chem. Commun., 2014, 50, 1465 RSC; (i) N. Thirupathi, M. H. Babu, V. Dwivedi, R. Kant and M. S. Reddy, Org. Lett., 2014, 16, 2908 CrossRef CAS; (j) J. Lei, X. Wu and Q. Zhu, Org. Lett., 2015, 17, 2322 CrossRef CAS; (k) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS; (l) S. Lang, Chem. Soc. Rev., 2013, 42, 4867 RSC; (m) G. Qiu and J. Wu, Chem. Commun., 2012, 48, 6046 RSC; (n) T. Fang, Q. Tan, Z. Ding, B. Liu and B. Xu, Org. Lett., 2014, 16, 2342 CrossRef CAS; (o) P.-L. Shao, J.-Y. Liao, Y. A. Ho and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 5435 CrossRef CAS; (p) H. Wang and B. Xu, Chin. J. Org. Chem., 2015, 35, 588 CrossRef CAS; (q) J. Wang, S. Luo, J. Li and Q. Zhu, Org. Chem. Front., 2014, 1, 1285 RSC; (r) S. Wang, L.-J. Yang, J.-L. Zeng, Y. Zheng and J.-A. Ma, Org. Chem. Front., 2015, 2, 1468 RSC; (s) L. Wang, J. Ferguson and F.-L. Zeng, Org. Biomol. Chem., 2015, 13, 11486 RSC; (t) Q. Zheng, Q. Ding, C. Wang, W. Chen and Y. Peng, Tetrahedron, 2016, 72, 952 CrossRef CAS.
- (a) J.-J. Cao, T.-H. Zhu, S.-Y. Wang, Z.-Y. Gu, X. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 6439 RSC; (b) J.-J. Cao, X. Wang, S.-Y. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 12892 RSC; (c) J.-J. Cao, T.-H. Zhu, Z.-Y. Gu, W.-J. Hao, S.-Y. Wang and S.-J. Ji, Tetrahedron, 2014, 70, 6985 CrossRef CAS.
- T.-H. Zhu, S.-Y. Wang, G.-N. Wang and S.-J. Ji, Org. Lett., 2014, 16, 1260 CrossRef CAS.
- (a) T.-H. Zhu, S.-Y. Wang, G.-N. Wang and S.-J. Ji, Chem.–Eur. J., 2013, 19, 5850 CrossRef CAS; (b) Y. Li, G. Qiu, Q. Ding and J. Wu, Tetrahedron, 2014, 70, 4652 CrossRef CAS; (c) T. Xiao, L. Li, G. Lin, Q. Wang, P. Zhang, Z.-W. Mao and L. Zhou, Green Chem., 2014, 16, 2418 RSC; (d) J. Liu, C. Fan, H. Yin, C. Qin, G. Zhang, X. Zhang, H. Yi and A. Lei, Chem. Commun., 2014, 50, 2145 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03216h |
| This journal is © The Royal Society of Chemistry 2016 |