Ni(ii)-catalyzed mono-selective ortho-arylation of unactivated aryl C–H bonds utilizing amino acids as a directing group†
Abstract
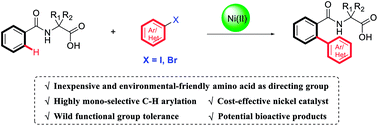
The nickel(II)-catalyzed ortho-arylation of unactivated C–H bonds utilizing amino acids as directing groups with aryl iodides or bromides as coupling electrophiles is described. This protocol features excellent mono-selectivity, good regioselectivity, and wide functional group tolerance. Additionally, the obtained products bearing a biaryl motif and an amino acid represent bioactive molecules with wide bioactivities.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations


 Please wait while we load your content...
Please wait while we load your content...