B(C6F5)3 catalysed 1,6-conjugate allylation of para-quinone methides: expedient access to allyl diarylmethanes†
Abstract
An effective method for the synthesis of unsymmetrical allyldiaryl methanes is portrayed. This protocol involves a Lewis acid catalysed 1,6-conjugate addition of allyltrimethyl silanes to para-quinone methides, which enabled us to synthesize a variety of unsymmetrical allyl diarylmethanes in good to excellent yields in a very short reaction period.
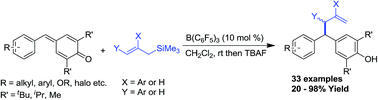
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations


 Please wait while we load your content...
Please wait while we load your content...