Ruthenium-catalyzed direct arylations with aryl chlorides
Abstract
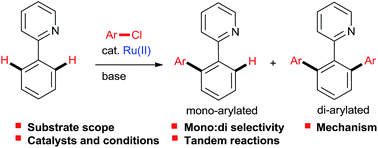
Aryl chlorides are readily available at lower cost than the corresponding bromides and iodides, but are much more challenging as substrates in metal-catalyzed cross-couplings. Using arenes as nucleophilic partners by means of C–H activation instead of arylorganometallics also carries significant cost and environmental advantages. Finally, Ru catalysts, characterized by lower metal cost, excellent chemoselectivity, and high activity in C–H functionalization even in “green solvents” such as water, compound the economic and environmental benefits of chelation-assisted direct arylation. The current state-of-the-art of the Ru-catalyzed direct aryl–aryl coupling with chloroarenes will be reviewed.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...