Copper-catalyzed N-formylation of amines with CO2 under ambient conditions†
Abstract
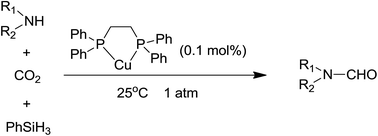
We carried out work on N-formylation of amines with CO2 and PhSiH3 to produce formamides catalyzed by a copper complex. It was found that the Cu(OAc)2–bis(diphenylphosphino)ethane (dppe) catalytic system was very efficient for these kind of reactions at room temperature and 1 atm CO2 with only 0.1 mol% catalyst loading.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...