Organocatalytic one-pot asymmetric synthesis of functionalized spiropyrazolones via a Michael-aldol sequential reaction†
Abstract
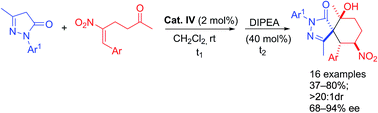
An efficient organocatalytic method was developed for synthesizing functionalized spiropyrazolone derivatives containing four contiguous stereogenic centres by using a Michael-aldol consecutive reaction. We applied a quinine-derived squaramide catalyst to catalyze a reaction between 3-methyl-1-aryl-2-pyrazolin-5-ones and (E)-5-nitro-6-aryl-hex-5-en-2-ones, realizing the desired spiropyrazolones in moderate-to-good yields (up to 80%) with good-to-excellent stereoselectivities (up to >20 : 1 dr, 94% ee).
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...