Rh(iii)-catalyzed C–H oxidative ortho-olefination of arenes using 7-azaindole as a directing group and utilization in the construction of new tetracyclic heterocycles containing a 7-azaindole skeleton†
Abstract
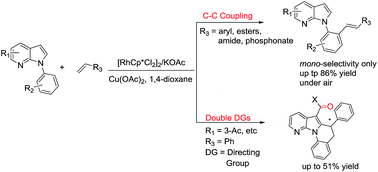
A Rh(III)-catalyzed C–H ortho-mono-olefination of aryls directed by 7-azaindoles was reported. This method opens up a novel pathway to synthesize complex 7-azaindole derivatives and interestingly, we observed an intramolecular cascade annulation via unexpected double directing groups (7-azaindole and carbonyl).
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...