Aqueous biphasic iron-catalyzed asymmetric transfer hydrogenation of aromatic ketones†
Abstract
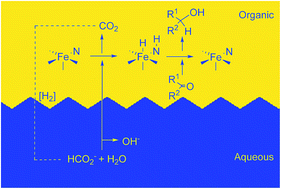
For the first time, an iron(II) catalyst is used in the biphasic asymmetric transfer hydrogenation (ATH) of ketones to enantioenriched alcohols employing water and potassium formate as the proton and hydride source, respectively. The precatalyst [FeCl(CO)(P–NH–N–P)]BF4 (P–NH–N–P = (S,S)-PPh2CH2CH2NHCHPhCHPhNCHCH2PPh2) in the organic phase with the substrate is activated by base to produce a system that rivals the best ruthenium biphasic ATH catalysts in activity but not enantioselectivity. Biorenewable 2-methyltetrahydrofuran as a cosolvent and biodegradable TWEEN80 as a surfactant were added to the reaction mixture to greatly decrease the mass-transfer limitations caused by the biphasic reaction mixture. The enantioselectivity of the reduction was as high as 76% depending on the substitution pattern of the arylketone employed. NMR studies verified the formation of an iron hydride [FeH(CO)(PPh2CH2CH2NHCHPhCHPhNCHCHPPh2)] intermediate as was observed in our 2-propanol-based ATH studies.
- This article is part of the themed collections: Editors’ collection: Catalytic Organic Transformations and Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...