Open Access Article
Open Access ArticlePalladium catalyzed Suzuki cross-coupling of benzyltrimethylammonium salts via C–N bond cleavage†
Tao
Wang
 ab,
Shuwu
Yang
b,
Silin
Xu
b,
Chunyu
Han
b,
Ge
Guo
b and
Junfeng
Zhao
*ab
ab,
Shuwu
Yang
b,
Silin
Xu
b,
Chunyu
Han
b,
Ge
Guo
b and
Junfeng
Zhao
*ab
aNational Research Center for Carbohydrate Synthesis, Key Laboratory of Chemical Biology, Jiangxi Province, China
bCollege of Chemistry & Chemical Engineering, Jiangxi Normal University, 99, Ziyang Road, Nanchang, Jiangxi 330022, P. R. China. E-mail: zhaojf@jxnu.edu.cn
First published on 9th March 2017
Abstract
A palladium catalyzed Suzuki cross-coupling for construction of Csp3–Csp2 bond via Csp3–N bond activation of benzyltrimethyl-ammonium salt is described. This reaction not only offered a highly efficient approach to diarylmethanes but also paved the way for the application of benzyltrimethylammonium salts in the palladium catalyzed cross coupling reactions.
Palladium catalyzed cross-coupling reactions between various electrophiles and nucleophilic metal reagents are highly practical methodologies for C–C bond formation.1 They are one of the significant accomplishments of last century's chemistry and their significance has been recognized by the 2010 Nobel Prize in Chemistry. Among these cross coupling reactions, the Suzuki reaction, the coupling between organoborane reagents and electrophiles, is the most popular one because of the inherent advantages of organoboron reagents, such as air- and moisture-stability, good functional group tolerance, low toxicity and wide availability. A variety of electrophiles such as aryl halides,2 triflates,3 mesylates/tosylates,4 esters,5 ethers,6 and phosphates7 have been developed for this reaction. Very recently, the transition metal catalyzed Suzuki coupling reactions involving the C–N bonds cleavage have attracted much attentions.8 Quaternary ammonium salts9 are the most important C–N bond containing electrophiles due to their wide availability and high reactivity. Macmillan and co-workers reported the first Ni catalyzed Suzuki cross-couplings of aryltrimethylammoniums.8i Watson extended this concept to benzyltrimethyl ammoniums and realized the stereospecific preparation of enantioenriched diarylethanes.8j However, both cases involved the using of air and moisture sensitive Ni catalysts which limited their practical application. Compared with Ni catalyzed reactions carried out under inert atmosphere, palladium catalyzed coupling reactions are relatively easy to handle. However, the palladium catalyzed cross-coupling reactions involving C–N bond cleavage of quaternary ammonium salts remained rare. Recent studies demonstrated that palladium could be used as valid catalyst for cross-coupling reaction of quaternary ammonium salts. Wang and co-workers reported a palladium catalyzed C–H arylation of oxazoles with aryltrimethyl-ammonium salts.10 Reeves developed the palladium catalyzed cross-coupling between aryltrimethyl-ammonium salts and Grignard reagents.11 However, palladium catalyzed Suzuki cross-coupling of quaternary ammonium salts is still kept untouched. In addition, considering the diversity of palladium catalyzed organic transformations, establishment of robust palladium catalyst systems for activating the C–N bond of quaternary ammonium salts would be valuable for exploring their application in organic synthesis. In such context, we herein reported the first palladium catalyzed Suzuki coupling with benzyltrimethylammoniums as the electrophile partner.
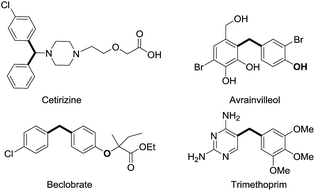
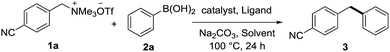
Diarylmethane derivatives have been widely used in pharmaceuticals and materials (Fig. 1).12 Very recently, Rhee disclosed that the synthesis of diarylmethanes could be accomplished by palladium catalyzed Suzuki cross-coupling of N,N-ditosylbenzylamines13via C–N bond activation. We envisioned that palladium catalyzed Suzuki cross-coupling of benzylammonium salts with aryl boronic acids would be an effective approach to diarylmethanes. It is initiated by investigating the crossing coupling of 4-cyanophenyl boronic acid with benzylammonium salt 1a, which is readily prepared quantitatively via methylation of 4-cyano-N,N-dimethylbenzylamine. As shown in Table 1, the reaction is sensitive to ligand. Only the monodentate PPh3 ligand afforded moderate yield of target diarylmethane accompanied by significant amount of homo coupling product of boronic acid when PdCl2 was used as the catalyst. Although further screening of palladium catalysts did not offer any improvement, a dramatic solvent affect was observed (Table 1, entries 13–18). In particularly, homo coupling of boronic acid, the notorious issue of Suzuki cross-coupling, could be suppressed significantly by using ethanol as the solvent. We were delighted to observe that the homo coupling could be suppressed completely when more ethanol was used (Table 1, entry 19). In turn, the yield of cross coupling product was increased up to quantitative. The similar reaction efficiencies were observed when potassium phenyltrifluoroborate and phenylboronic acid pinacol ester were used as the boron reagent (Table 1, entry 20 & 21).
| Entry | Catalyst (3 mol%) | Ligand (10 mol%) | Solvent | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), catalyst (3 mol%), ligand (10 mol%) and Na2CO3 (2 equiv.) in solvent (1 mL) at 100 °C. Mixture: toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 9 DMSO = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b GC yield, isolated yield was indicated parentheses.
c EtOH (3 mL).
d Potassium phenyltrifluoroborate was used in place of boronic acid.
e Phenylboronic acid pinacol ester as the boron reagent. 1.
b GC yield, isolated yield was indicated parentheses.
c EtOH (3 mL).
d Potassium phenyltrifluoroborate was used in place of boronic acid.
e Phenylboronic acid pinacol ester as the boron reagent.
|
||||
| 1 | PdCl2 | — | Mixture | — |
| 2 | PdCl2 | dppf | Mixture | — |
| 3 | PdCl2 | dppp | Mixture | — |
| 4 | PdCl2 | Xantphos | Mixture | Trace |
| 5 | PdCl2 | PPh3 | Mixture | 67(65) |
| 6 | Pd(acac)2 | PPh3 | Mixture | 65 |
| 7 | Pd2(dba)3 | PPh3 | Mixture | 63 |
| 8 | Pd(TFA)2 | PPh3 | Mixture | 57 |
| 9 | PdCl2(PPh3)2 | PPh3 | Mixture | 55 |
| 10 | Pd(PPh3)4 | PPh3 | Mixture | 54(50) |
| 11 | PdCl2(dppf)2 | PPh3 | Mixture | 50 |
| 12 | Pd(OAc)2 | PPh3 | Mixture | 20 |
| 13 | PdCl2 | PPh3 | PhMe | 34 |
| 14 | PdCl2 | PPh3 | DMSO | 18 |
| 15 | PdCl2 | PPh3 | DMF | 54 |
| 16 | PdCl2 | PPh3 | CH3CN | 43 |
| 17 | PdCl2 | PPh3 | THF | 32 |
| 18 | PdCl2 | PPh3 | EtOH | 75 |
| 19c | PdCl2 | PPh3 | EtOH | 99(98) |
| 20d | PdCl2 | PPh3 | EtOH | 97 |
| 21e | PdCl2 | PPh3 | EtOH | 96 |
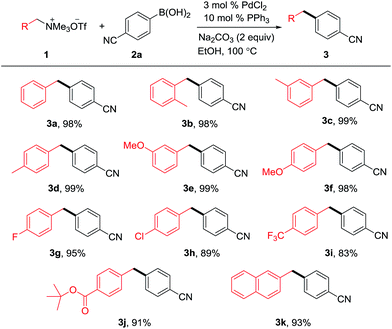
With the optimized reaction conditions in hand, we next set out to evaluate the generality of benzylammonium salts with 4-cyanophenyl boronic acid as a model substrate. As shown in Scheme 1, a broad substrate scope of benzylammonium salts was observed. It seems that the electronic effect and the steric effect of the substituents on the benzylammonium salts have little effect on the reaction efficiency. No matter electron-donating (3b–f) or -withdrawing (3g–j) groups on the phenyl ring of benzylammonium salts, good to excellent yields were obtained. The ortho methyl substituent could be tolerated (3b). Notably, the C–N bond could be selectively cleaved in the presence of ether (3e & 3f), fluoro (3g) and chloro (3h) groups albeit bromo and iodo-substituted benzylammonium salts did not offer the target cross coupling products.
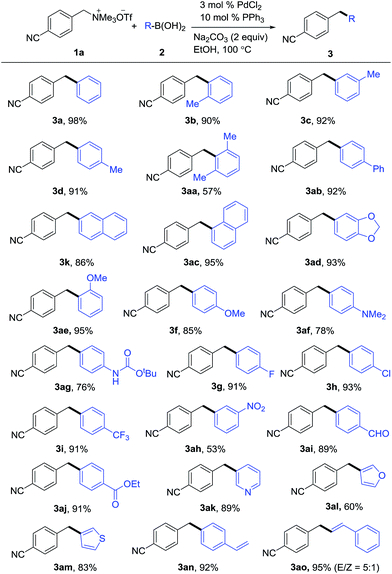
The substrate scope of boronic acids had also been investigated and the results are summarized in Scheme 2. Moderate electron-withdrawing and -donating groups were tolerated while their stronger congeners cause slight decrease in yield. Even the steric demanding 2,6-dimethyl substituents were tolerated (3aa). It is noted that a series of functional groups such as carbonate (3ag), aldehyde (3ai) and ester (3aj) were compatible. In addition, diarylmethanes containing heterocycles such as pyridine (3ak), furan (3al) and thiophene (3am) could also be prepared by this method. Styrylboronic acid also proceeded smoothly to furnish the cross coupling product in excellent yield (3ao). No evidence of Heck coupling was observed when (4-vinylphenyl)boronic acid was employed as the substrate (3an). Interestingly, for some cases (3a–d, 3f–i), we could get the same products with that of Scheme 1 from different set of starting materials by employing the standard reaction conditions. This advantage will be more prominent when one of the starting material is more expensive or not available.
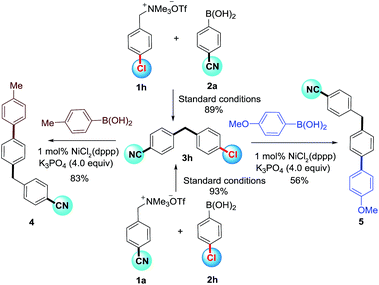
A significant functional group orthogonality to chloride, fluoride, ether and amine for benzylammonium salts as well as boronic acids was observed in this methodology. For example, the chloro group was kept intact in both cases (3h) and thus affording an opportunity for further functionalization. This feature was exemplified by sequential Suzuki-cross couplings (Scheme 3).
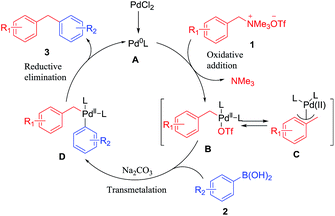
A plausible reaction mechanism was proposed in Scheme 4. The oxidative addition of Pd(0) A formed in situ, with benzyltrimethylammonium salt 1 produced C–Pd complex B with the release of trimethylamine. Followed by a transmetalation with boronic acid, the palladium complex B afforded a new complex D, which upon reductive elimination furnished the cross coupling product 3.
In summary, we have developed the first palladium catalyzed Suzuki cross-coupling reaction of quaternary ammonium salts. This reaction represented the first example of palladium catalyzed Csp3–Csp2 coupling of benzyltrimethylammonium salts via C–N bond activation. It not only offered an effective synthetic strategy to diarylmethane derivatives but also paved the way for the unprecedented palladium catalyzed cross-coupling of benzyltrimethyl ammonium salts. Further application of the catalyst system to other palladium catalyzed cross-coupling reactions of benzyltrimethyl ammonium salts are undergoing in our laboratory and the results will be reported in due course.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21462023, 21562026) and the Natural Science Foundation of Jiangxi Province (20143ACB20007, 20161BAB203085).References
- C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef CAS.
- (a) X. Bei, H. W. Turner, W. H. Weinberg, A. S. Guram and J. L. Petersen, J. Org. Chem., 1999, 64, 6797 CrossRef; (b) J. P. Wolfe, R. A. Singer, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550 CrossRef CAS; (c) G. A. Grasa, A. C. Hillier and S. P. Nolan, Org. Lett., 2001, 3, 1077 CrossRef CAS; (d) N. E. Leadbeater and M. Marco, Org. Lett., 2002, 4, 2973 CrossRef CAS; (e) G. A. Molander and B. Biolatto, Org. Lett., 2002, 4, 1867 CrossRef CAS; (f) O. Navarro, R. A. Kelly and S. P. Nolan, J. Am. Chem. Soc., 2003, 125, 16194 CrossRef CAS; (g) N. Marion, O. Navarro, J. Mei, E. D. Stevens, N. M. Scott and S. P. Nolan, J. Am. Chem. Soc., 2006, 128, 4101 CrossRef CAS; (h) K. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3358 CrossRef CAS PubMed; (i) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed.
- A. F. Littke, C. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020 CrossRef CAS.
- (a) M. K. Lakshman, P. F. Thomson, M. A. Nuqui, J. H. Hilmer, N. Sevova and B. Boggess, Org. Lett., 2002, 4, 1479 CrossRef CAS; (b) H. N. Nguyen, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 11818 CrossRef CAS; (c) K. W. Quasdorf, M. Riener, K. V. Petrova and N. K. Garg, J. Am. Chem. Soc., 2009, 131, 17748 CrossRef CAS.
- (a) D. Bouyssi, V. Gerusz and G. Balme, Eur. J. Org. Chem., 2002, 2445 CrossRef CAS; (b) H. Tatamidani, F. Kakiuchi and N. Chatani, Org. Lett., 2004, 6, 3597 CrossRef CAS; (c) B.-T. Guan, Y. Wang, B.-J. Li, D.-G. Yu and Z.-J. Shi, J. Am. Chem. Soc., 2008, 130, 14468 CrossRef CAS PubMed.
- (a) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866 CrossRef CAS; (b) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS; (c) M. Tobisu and N. Chatani, Acc. Chem. Res., 2015, 48, 1717 CrossRef CAS; (d) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486 CrossRef CAS; (e) D.-G. Yu, B.-J. Li, S.-F. Zheng, B.-T. Guan, B.-Q. Wang and Z.-J. Shi, Angew. Chem., Int. Ed., 2010, 49, 4566 CrossRef CAS; (f) K. Huang, D.-G. Yu, S.-F. Zheng, Z.-H. Wu and Z.-J. Shi, Chem.–Eur. J., 2011, 17, 786 CrossRef CAS.
- (a) U. S. Larsen, L. Martiny and M. Begtrup, Tetrahedron Lett., 2005, 46, 4261 CrossRef CAS; (b) L. Pedzisa, I. W. Vaughn and R. Pongdee, Tetrahedron Lett., 2008, 49, 4142 CrossRef CAS.
- (a) K. Ouyang, W. Hao, W. X. Zhang and Z. Xi, Chem. Rev., 2015, 115, 12045 CrossRef CAS; (b) T. Saeki, E.-C. Son and K. Tamao, Org. Lett., 2004, 6, 617 CrossRef CAS; (c) J. Liu and M. J. Robins, Org. Lett., 2004, 6, 3421 CrossRef CAS; (d) K. R. Buszek and N. Brown, Org. Lett., 2007, 9, 707 CrossRef CAS; (e) Z. Peng, G. Hu, H. Qiao, P. Xu, Y. Gao and Y. Zhao, J. Org. Chem., 2014, 79, 2733 CrossRef CAS PubMed; (f) Y. Zhao and V. Snieckus, Org. Lett., 2014, 16, 3200 CrossRef CAS; (g) J. Liu and M. J. Robins, Org. Lett., 2005, 7, 1149 CrossRef CAS; (h) M. L. Duda and F. E. Michael, J. Am. Chem. Soc., 2013, 135, 18347 CrossRef CAS; (i) S. B. Blakey and D. W. C. MacMillan, J. Am. Chem. Soc., 2003, 125, 6046 CrossRef CAS; (j) P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 280 CrossRef CAS; (k) D. M. Shacklady-McAtee, K. M. Roberts, C. H. Basch, Y. G. Song and M. P. Watson, Tetrahedron, 2014, 70, 4257 CrossRef CAS; (l) S. Ueno, N. Chatani and F. Kakiuchi, J. Am. Chem. Soc., 2007, 129, 6098 CrossRef CAS; (m) T. Koreeda, T. Kochi and F. Kakiuchi, J. Am. Chem. Soc., 2009, 131, 7238 CrossRef CAS; (n) M.-K. Zhu, J.-F. Zhao and T.-P. Loh, Org. Lett., 2011, 13, 6308 CrossRef CAS; (o) N. A. Weires, E. L. Baker and N. K. Garg, Nat. Chem., 2016, 8, 75 CrossRef CAS; (p) P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 519 CrossRef CAS; (q) T. N. Uehara, J. Yamaguchi and K. Itami, Asian J. Org. Chem., 2013, 2, 938 CrossRef CAS; (r) M.-B. Li, Y. Wang and S.-K. Tian, Angew. Chem., Int. Ed., 2012, 51, 2968 CrossRef CAS; (s) M. Tobisu, K. Nakamura and N. Chatani, J. Am. Chem. Soc., 2014, 136, 5587 CrossRef CAS.
- (a) E. Wenkert, A. L. Han and C. J. Jenny, Chem. Commun., 1988, 975 RSC; (b) L. G. Xie and Z. X. Wang, Angew. Chem., Int. Ed. Engl., 2011, 50, 4901 CrossRef CAS; (c) W. J. Guo and Z. X. Wang, Tetrahedron, 2013, 69, 9580 CrossRef CAS; (d) X. Yang and Z.-X. Wang, Organometallics, 2014, 33, 5863 CrossRef CAS; (e) X. Q. Zhang and Z. X. Wang, Org. Biomol. Chem., 2014, 12, 1448 RSC; (f) J. Hu, H. Sun, W. Cai, X. Pu, Y. Zhang and Z. Shi, J. Org. Chem., 2016, 81, 14 CrossRef CAS; (g) H. Zhang, S. Hagihara and K. Itami, Chem.–Eur. J., 2015, 21, 16796 CrossRef CAS; (h) T. Moragas, M. Gaydou and R. Martin, Angew. Chem., Int. Ed., 2016, 55, 5053 CrossRef CAS; (i) Y. Z. Lei, R. Zhang, L. J. Wu, Q. Wu, H. Mei and G. X. Li, Appl. Organomet. Chem., 2014, 28, 310 CrossRef CAS; (j) H. J. Davis, M. T. Mihai and R. J. Phipps, J. Am. Chem. Soc., 2016, 138, 12759 CrossRef CAS; (k) X.-Q. Zhang and Z.-X. Wang, J. Org. Chem., 2012, 77, 3658 CrossRef CAS; (l) D. Wu, J.-L. Tao and Z.-X. Wang, Org. Chem. Front., 2015, 2, 265 RSC.
- F. Zhu, J. L. Tao and Z. X. Wang, Org. Lett., 2015, 17, 4926 CrossRef CAS.
- J. T. Reeves, D. R. Fandrick, Z. Tan, J. J. Song, H. Lee, N. K. Yee and C. H. Senanayake, Org. Lett., 2010, 12, 4388 CrossRef CAS.
- (a) A. V. Cheltsov, M. Aoyagi, A. Aleshin, E. C.-W. Yu, T. Gilliland, D. Zhai, A. A. Bobkov, J. C. Reed, R. C. Liddington and R. Abagyan, J. Med. Chem., 2010, 53, 3899 CrossRef CAS; (b) S. Messaoudi, A. Hamze, O. Provot, B. Tréguier, J. Rodrigo De Losada, J. Bignon, J.-M. Liu, J. Wdzieczak-Bakala, S. Thoret, J. Dubois, J.-D. Brion and M. Alami, ChemMedChem, 2011, 6, 488 CrossRef CAS; (c) B. Liegault, J.-L. Renaud and C. Bruneau, Chem. Soc. Rev., 2008, 37, 290 RSC.
- S. Yoon, M. C. Hong and H. Rhee, J. Org. Chem., 2014, 79, 4206 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra02549a |
| This journal is © The Royal Society of Chemistry 2017 |