L-Isoleucine derived bifunctional phosphine catalyses asymmetric [3 + 2]-annulation of allenyl-esters and -ketones with ketimines†
Muthukumar G.
Sankar
a,
Miguel
Garcia-Castro
a,
Christopher
Golz
b,
Carsten
Strohmann
b and
Kamal
Kumar
*a
aMax-Planck-Institut für Molekulare Physiologie, Abteilung Chemische Biologie, Otto-Hahn-Straße 11, 44227 Dortmund, Germany. E-mail: kamal.kumar@mpi-dortmund.mpg.de
bFakultät Chemie und Chemische Biologie, Technische Universität Dortmund, Otto-Hahn-Straße 6, 44221 Dortmund, Germany
First published on 8th June 2016
Abstract
The zwitterionic 1,3-dipoles generated by the addition of a bifunctional L-isoleucine derived N-acylaminophosphine to allenic esters as well as ketones were successfully trapped with isatin derived ketimines in a [3 + 2]-annulation reaction to deliver 3,2′-dihydropyrrolyl spirooxindoles in high yields (up to 88%) and with excellent enantioselectivities (up to >99%). The asymmetric annulation reaction provides a facile access to biologically relevant small molecules embodying the natural product spirocyclic core.
Introduction
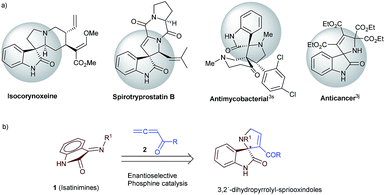
The biological relevance of the core-structures representing small molecule collections is an important criterion that influences their potential to deliver disease or biology modulating chemical entities.1 The privileged spirooxindole framework often occurs in complex and diversely bioactive natural products as well as in therapeutically useful synthetic small molecules (Scheme 1a).2,3 Asymmetric dipolar annulation reactions that introduce different spiro-ring-systems to an oxindole scaffold are therefore highly desired.4 Asymmetric phosphine catalyzed dipolar annulation of electron-poor allenes, in particular allene esters with various electron deficient substrates is one of the important transformations that afford a range of carbo- and heterocycles and thereby contribute valuable building blocks finding applications in synthesis of a wide range of bioactive natural products and medicinally important substances.5,6 Although several phosphine-catalyzed reactions have been developed for the syntheses of various acyclic and cyclic motifs, only a limited number of asymmetric variants have evolved.5c In particular, the asymmetric annulation reactions between allene derived zwitterions and imines remain limited to aldimines6b–e,g,l–q and the potential of ketimines in asymmetric phosphine catalysis to build diverse ring-systems with quaternary centres remain underexplored. Compared to the aldimines, ketimines are typically less reactive and are challenging substrates for enantioselective transformations.7 We envisioned that the annulation reactions employing isatin derived ketimine (1, Scheme 1b), which are readily accessible and stable compounds,8 can provide rapid and concise access to biologically relevant class of spirooxindole small molecules.Only recently, chemists have begun to explore the potential of isatin derived ketimines in organic synthesis. In 2012, Chi et al. reported an N-heterocyclic carbene (NHC)-catalyzed enantioselective annulation reaction of isatin N-Boc ketimines with enals to build spirocyclic oxindole-γ-lactams.9 In 2013, Kumar and coworkers have utilized isatinimine for phosphine mediated dipolar annulation reaction of Huisgen zwitterions to generate dihydro-1H-1,2,4-triazoles.8b In 2014, Ye et al. reported the synthesis of spirocyclic oxindolo-β-lactams from isatinimines via NHC-catalyzed reaction of ketenes.10 Recently, Ye et al. reported NHC-catalyzed [4 + 2]-annulation of α,β-unsaturated carboxylic acid with isatinimine to generate spirocylic oxindolodihydropyridinones.11 Enders et al. had described the synthesis of a new class of pyrrolidinyl-dispirooxindoles via an organocatalytic Mannich/Boc-deprotection/aza-Michael sequence from isatinimine.12 Concurrently, Li and coworkers introduced a formal asymmetric [3 + 2]-annulation of 1,4-dithiane-2,5-diol with isatinimines to produce 4-thiazolidinone.13 Marinetti and co-workers had recently reported a binepine catalysed asymmetric [3 + 2]-annulation reaction of allene esters with 3-arylidene indolin-2-ones to generate cyclopentene fused spirooxindoles.6k Herein, we report an efficient phosphine catalyzed asymmetric [3 + 2]-annulation reaction of allenyl carbonyl compounds with electron-deficient isatin derived ketimines affording a facile entry to 3,2′-dihydropyrrolyl spirooxindoles (Scheme 1b).14
Results and discussion
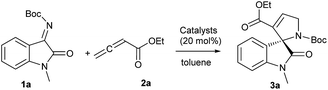
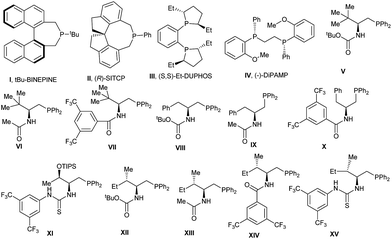
We began our investigation using N-Boc isatinimine 1a and ethyl 2,3-butadienoate 2a as model substrates for asymmetric dipolar annulation reaction (Table 1 and Fig. 1). The binepine monophosphine catalyst (I) that had been successfully used by Fu et al. in an enantioselective [4 + 2] annulation reaction of allene esters 1 with aldimines,6q provided only moderate yield of 3a with very low enantiomeric excess (ee) (entry 1). Spiro-phosphine (R)-SITCP (II) that Shi et al. had successfully employed in a [3 + 2] annulation reaction of allene esters with olefins,15 though provided adduct 3a in good yield, but failed to provide good asymmetric induction (entry 2). While (S,S)-Et-DUPHOS (III) failed to catalyse the reaction (entry 3), the DiPAMP (IV) gave a moderate yield for 3a and with only low ee (entry 4). With these disappointing results, we resorted to bifunctional N-acylaminophosphines with the hope that H-bond donating ability of these phosphines might positively influence the desired enantioselective annulation.6g,16L-tert-Leucine derived aminophosphines (V–VII) afforded spirooxindole 3a in moderate yield but with slightly improved enantiomeric excess (entry 5–7). We were pleased to observe that L-phenyl alanine derived aminophosphine catalyst (VIII) enhanced the yield as well as enantiomeric excess of product (ee, entry 8). However, other similar catalysts IX and X showed very poor results (entry 9 & 10). L-Threonine based catalyst XI also failed to steer the reaction efficiently and enantioselectively (entry 11). Some encouraging results were finally obtained with L-isoleucine based aminophosphines when catalysts XII & XIII provided adduct 3a in good yields and with moderate ee's (entry 12 & 13). Catalyst XIV supporting bis(3,5-trifluoro-methyl)benzamide displayed highly encouraging results (64% yield, 70% ee) (entry 14). Catalyst XV with a stronger H-bond donating thiourea functionality however failed to improve the results (entry 15). Further reaction condition optimization (entries 16–20) with catalyst XIV revealed that the reaction at −40 °C and in toluene using 0.035 M solution of isatinimine delivered the best result for the formation of 3a (84% yield and 97% ee, entry 19). Further lowering the temperature to −78 °C did not produce any reaction (entry 20).| Entryb | Catalyst | Solvent | Temp. (°C) | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 equiv., 0.06 M), 2a (1.2 equiv.), cat. (20 mol%), 12 h, rt. b Reaction was monitored by TLC analysis as well as HPLC-MS. c 0.035 M solution of 1a in toluene was used. d Reaction is very sluggish. | |||||
| 1 | I | Toluene | 25 | 49 | −16 |
| 2 | II | Toluene | 25 | 70 | +16 |
| 3 | III | Toluene | 25 | — | N/A |
| 4 | IV | Toluene | 25 | 56 | −12 |
| 5 | V | Toluene | 25 | 43 | −25 |
| 6 | VI | Toluene | 25 | 61 | −30 |
| 7 | VII | Toluene | 25 | 68 | −38 |
| 8 | VIII | Toluene | 25 | 55 | −58 |
| 9 | IX | Toluene | 25 | 48 | +4 |
| 10 | X | Toluene | 25 | 36 | N/A |
| 11 | XI | Toluene | 25 | 65 | −14 |
| 12 | XII | Toluene | 25 | 71 | −40 |
| 13 | XIII | Toluene | 25 | 49 | −33 |
| 14 | XIV | Toluene | 25 | 64 | −70 |
| 15 | XV | Toluene | 25 | 41 | +57 |
| 16 | XIV | DCM | 25 | 62 | −59 |
| 17 | XIV | Dioxane | 25 | 0 | — |
| 18 | XIV | Toluene | −20 | 68 | −91 |
| 19 | XIV | Toluene c | −40 | 84 | −97 |
| 20 | XIV | Toluene | −78d | — | — |
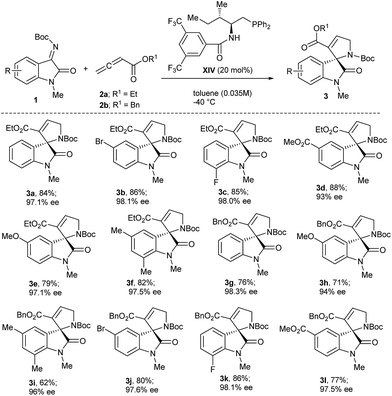
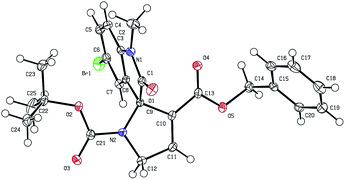
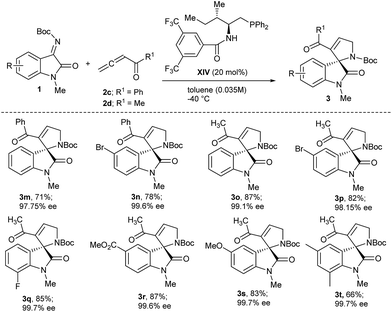
Under the optimized reaction conditions for asymmetric [3 + 2] annulation reaction, the scope of the reaction was investigated by using a variety of substituted isatinimines 1 and different allene esters 2 (Scheme 2). Pleasingly, all the reactions proceeded smoothly to furnish the corresponding products 3 in high yields and with excellent enantioselectivities (Scheme 2). Isatinimines with electron-withdrawing functionalities afforded spirooxindoles (3b–3d) in high yields and better ee's as compared to 3a. Meanwhile adducts obtained from ketimines with electron rich aryl rings maintained higher ee's albeit with slightly decreased yields (3e and 3f). The annulation reaction of 1 with zwitterion derived from benzyl allenoate 2b also ran smoothly to offer [3 + 2] adducts 3g–3l in high yields and with excellent enantiomeric excess (Scheme 2). The absolute stereochemistry of all the spirooxindole products 3 was unambiguously assigned on the basis of a single crystal X-ray analysis of 3j (Fig. 2).17
Phosphine catalysis has more often engaged the allene esters as substrates of the zwitterions and the corresponding allenic ketones have only rarely been explored in the zwitterionic annulation reactions.18 We employed allenyl ketones 2c and 2d in the [3 + 2]-annulation reaction with isatinimines 1 (Scheme 3). With allenyl phenyl ketone 2c, the annulation reaction of isatinimines 1 performed well to afford adducts 3m and 3n in high yields and with very good enantioselectivities (Scheme 3). The acetyl allene, which has a very low boiling point, also sustained well with the asymmetric annulation reaction conditions and afforded the corresponding spiro-adduct in very good yields and with excellent ee's (3o–3t).
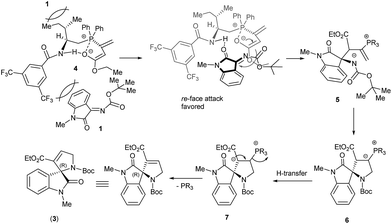
The role of Brønsted acid function in the aminophosphine catalysts XIV to steer the stereoselectivity of the reaction is obvious and plausibly helps in stabilizing the enolate zwitterion 4 with the help of H-bonding as well as O–P interaction (Scheme 4).13 The amino acid backbone as well as the bulky benzamide function are the key steric blocks that steer a highly facially discriminative approach of isatinimine as depicted in Scheme 4. We assume that the lactam function of oxindole might also interact by hydrogen bonding and thereby exposing the re-face of ketimine for an attack of zwitterion and leading to intermediate 5. Addition of amide function in 5 to the vinyl phosphonium moiety closes the ring (6). A proton transfer leading to enolate 7 is followed by the phosphine elimination to provide the spirooxindole adduct 3.
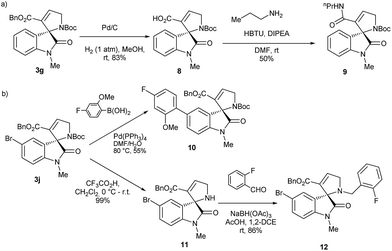
The synthetic utility of the enantio-enriched spirooxindoles 3 in delivering more enantiopure small molecules based on a natural product core-structure was further investigated as depicted in Scheme 5. Debenzylation of the spirooxindole 3g with Pd/C and under hydrogen atmosphere delivered the spirooxindole 8 in good yield and with a carboxylic acid as synthetic handle for parallel synthesis in the construction of a focused compound library (Scheme 5a). For instance, a coupling reaction of 8 with n-propylamine using HBTU as coupling reagent provided the amide 9. Suzuki coupling of the bromoaryl function in 3j under standard conditions afforded the aryl substituted spirooxindole 10 (Scheme 5b). Acid mediated removal of N-Boc group afforded the free amine 11 which reacted well with 2-fluoro-benzaldehyde under reductive amination condition to furnish compound 12 and thus demonstrating the amenability of the substituted spirooxindoles 3 in compound collection synthesis.
Conclusions
In conclusion, we have developed a highly enantioselective [3 + 2]-annulation reaction of isatin-derived ketimines as the electrophiles with zwitterions generated from allenyl esters as well as allenyl ketones. The key to success in this asymmetric transformation was the catalysis performed by L-isoleucine derived bifunctional N-acylaminophosphine. This catalytic protocol allows a rapid and efficient access to construct enantiopure small molecules with a dihydropyrrolyl-spirooxindole framework.Experimental section
General information
All the chemicals were purchased from Aldrich, Acros, or Alfa and were used without further purification. Reactions were carried out in standard glassware or a Radleys Carousel 12 parallel reactor. Thin layer chromatography (TLC) was performed on Merck silica gel 60F254 aluminum sheet. Column chromatography (CC) purifications were carried out using silica gel (Acros Organics, particle size 35–70 μm) was used. Solvents used for CC are commercially available. Fourier transform infrared spectroscopy (FT-IR) data were obtained from Bruker Tensor 27 spectrometer (ATR, neat) and are reported in terms of frequency of absorption. 1H and 13C-NMR spectroscopic data were recorded on a Varian Mercury VX 400 or Varian 500-inova500 spectrometer at RT. 1H and 13C-NMR spectra were calibrated to the solvent signals of CDCl3 (=7.26 and 77.00 ppm); multiplicities are indicated brs (broadened singlet), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), dd (doublets of a doublet); coupling constants (J) are given in Hertz (Hz). LC/MS analysis were done using an Agilent 6150 single quadrupole (SQ) mass spectrometer coupled to an Agilent 1290 Infinity LC System. High resolution mass spectra were recorded on a LTQ Orbitrap mass spectrometer coupled to an Acceka HPLC-System (HPLC column: Hypersyl GOLD, 50 mm × 1 mm, particle size 1.9 μm, ionization method: electron spray ionization). Optical rotations were measured in a Schmidt + Haensch Polartronic HH8 polarimeter. The enantiomeric excesses were determined by Agilent-1100 series HPLC system using a chiral stationary phase column (column: CHIRALPAK IC, CHIRALPAK IA, eluent: (DCM/EtOH = 100/2), iso-hexane). Chemical yields refer to pure isolated substances.General procedure for the [3 + 2]-dipolar annulation reaction
To a stirred solution of isatinimine 1 (1.0 equiv.) and allene 2 (1.2 equiv.) in degassed toluene (0.035 M of 1) was added the isoleucine derived bifunctional aminophosphine XIV (20 mol%) at −40 °C and the reaction mixture was continued to stir at −40 °C overnight (12 h). The reaction was monitored by TLC using Pet.ether/ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. After completion, the reaction mixture was concentrated to give the crude compound that was purified by flash column chromatography on silicagel using 35–60% ethyl acetate in petroleum ether as gradient eluent and afforded the spirooxindole 3.
1) as eluent. After completion, the reaction mixture was concentrated to give the crude compound that was purified by flash column chromatography on silicagel using 35–60% ethyl acetate in petroleum ether as gradient eluent and afforded the spirooxindole 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. After completion, the reaction mixture was concentrated to give the crude compound that was purified by flash column chromatography on silicagel using 35–60% ethyl acetate in petroleum ether as gradient eluent to give the spirooxindole 3a; yield: 84%; ee = 97.1%; [α]20D = −19.8° (c 1.17, CH2Cl2); IR νmax/cm−1 3058, 2978, 2934, 1788, 1721, 1703; 1H NMR (5
1) as eluent. After completion, the reaction mixture was concentrated to give the crude compound that was purified by flash column chromatography on silicagel using 35–60% ethyl acetate in petroleum ether as gradient eluent to give the spirooxindole 3a; yield: 84%; ee = 97.1%; [α]20D = −19.8° (c 1.17, CH2Cl2); IR νmax/cm−1 3058, 2978, 2934, 1788, 1721, 1703; 1H NMR (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.30 (td, J = 7.6, 1.5 Hz, 1H), 7.13 (t, J = 2.2 Hz, 1H), 7.08* (t, J = 2.2 Hz, 0.2H), 7.06–7.03 (m, 1H), 7.00 (td, J = 7.4, 0.9 Hz, 1H), 6.97* (td, J = 7.4, 0.9 Hz, 0.2H), 6.83* (d, J = 7.7 Hz, 0.3H), 6.79 (d, J = 7.7 Hz, 1H), 4.62 (dd, J = 18.3, 2.2 Hz, 1H), 4.56 (dd, J = 18.3, 2.2 Hz, 1H), 4.48* (dd, J = 18.1, 2.1 Hz, 0.2H), 4.05–3.86 (m, 2H), 3.28* (s, 0.5H), 3.23 (s, 3H), 1.36 (s, 1.5H), 1.07 (s, 9H), 1.09–0.99 (m, 3H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 174.24, 161.02, 152.56, 144.88*, 144.81, 140.20*, 140.06, 134.87*, 134.78, 134.68*, 130.28*, 129.67, 129.44, 129.09*, 128.67*, 122.87, 122.76, 122.74*, 122.59*, 108.29*, 108.02*, 107.86, 98.42, 83.41*, 80.95, 80.88*, 72.61, 60.99, 60.93*, 54.31*, 54.02, 53.62*, 28.54, 28.29*, 28.01, 27.89, 26.87*, 26.61, 14.01, 13.96*; HRMS [ESI]: calculated for C20H25N2O5 [M + H+]: 373.17580, found: 373.17624; RT = 21.1 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1
1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.30 (td, J = 7.6, 1.5 Hz, 1H), 7.13 (t, J = 2.2 Hz, 1H), 7.08* (t, J = 2.2 Hz, 0.2H), 7.06–7.03 (m, 1H), 7.00 (td, J = 7.4, 0.9 Hz, 1H), 6.97* (td, J = 7.4, 0.9 Hz, 0.2H), 6.83* (d, J = 7.7 Hz, 0.3H), 6.79 (d, J = 7.7 Hz, 1H), 4.62 (dd, J = 18.3, 2.2 Hz, 1H), 4.56 (dd, J = 18.3, 2.2 Hz, 1H), 4.48* (dd, J = 18.1, 2.1 Hz, 0.2H), 4.05–3.86 (m, 2H), 3.28* (s, 0.5H), 3.23 (s, 3H), 1.36 (s, 1.5H), 1.07 (s, 9H), 1.09–0.99 (m, 3H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 174.24, 161.02, 152.56, 144.88*, 144.81, 140.20*, 140.06, 134.87*, 134.78, 134.68*, 130.28*, 129.67, 129.44, 129.09*, 128.67*, 122.87, 122.76, 122.74*, 122.59*, 108.29*, 108.02*, 107.86, 98.42, 83.41*, 80.95, 80.88*, 72.61, 60.99, 60.93*, 54.31*, 54.02, 53.62*, 28.54, 28.29*, 28.01, 27.89, 26.87*, 26.61, 14.01, 13.96*; HRMS [ESI]: calculated for C20H25N2O5 [M + H+]: 373.17580, found: 373.17624; RT = 21.1 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in isohexane, flow rate: 0.5 mL min−1).
50) in isohexane, flow rate: 0.5 mL min−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.43 (dd, J = 8.2, 2.0 Hz, 1H), 7.40* (dd, J = 8.2, 2.0 Hz, 0.3H), 7.18 (d, J = 2.0 Hz, 1H), 7.15* (d, J = 1.9 Hz, 0.3H), 7.15 (t, J = 2.1 Hz, 1H), 7.10* (t, J = 2.1 Hz, 0.3H), 6.71* (d, J = 8.3 Hz, 0.3H), 6.68 (d, J = 8.2 Hz, 1H), 4.63 (dd, J = 18.4, 2.2 Hz, 1H), 4.61* (dd, J = 18.2, 2.1 Hz, 0.3H), 4.56 (dd, J = 18.4, 2.1 Hz, 1H), 4.47* (dd, J = 18.2, 2.1 Hz, 0.3H), 4.06–3.93 (m, 2H), 3.26* (s, 0.8H), 3.22 (s, 3H), 1.38 (s, 2H), 1.11 (s, 9H), 1.12–1.06 (m, 3H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 173.72, 173.68*, 160.88*, 160.83, 152.31, 151.91*, 144.06*, 143.92, 140.66*, 140.50, 134.41*, 134.36, 132.46*, 132.42, 131.48, 126.20, 126.05*, 115.21, 115.12*, 109.77*, 109.30, 81.35, 81.28*, 72.40, 61.20, 61.13*, 54.35*, 54.12, 28.54, 28.07, 26.96*, 26.72, 14.07, 14.02*; HRMS [ESI]: calculated for C20H24BrN2O5 [M + H+]: 451.08631, found: 451.08619; calculated for C20H2481BrN2O5 [M + H+]: 453.08426, found: 453.08414; RT = 51.9 min (chiral HPLC, chiralpak IC-column and using 30% of mixture of EtOH–DCM (1
1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.43 (dd, J = 8.2, 2.0 Hz, 1H), 7.40* (dd, J = 8.2, 2.0 Hz, 0.3H), 7.18 (d, J = 2.0 Hz, 1H), 7.15* (d, J = 1.9 Hz, 0.3H), 7.15 (t, J = 2.1 Hz, 1H), 7.10* (t, J = 2.1 Hz, 0.3H), 6.71* (d, J = 8.3 Hz, 0.3H), 6.68 (d, J = 8.2 Hz, 1H), 4.63 (dd, J = 18.4, 2.2 Hz, 1H), 4.61* (dd, J = 18.2, 2.1 Hz, 0.3H), 4.56 (dd, J = 18.4, 2.1 Hz, 1H), 4.47* (dd, J = 18.2, 2.1 Hz, 0.3H), 4.06–3.93 (m, 2H), 3.26* (s, 0.8H), 3.22 (s, 3H), 1.38 (s, 2H), 1.11 (s, 9H), 1.12–1.06 (m, 3H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 173.72, 173.68*, 160.88*, 160.83, 152.31, 151.91*, 144.06*, 143.92, 140.66*, 140.50, 134.41*, 134.36, 132.46*, 132.42, 131.48, 126.20, 126.05*, 115.21, 115.12*, 109.77*, 109.30, 81.35, 81.28*, 72.40, 61.20, 61.13*, 54.35*, 54.12, 28.54, 28.07, 26.96*, 26.72, 14.07, 14.02*; HRMS [ESI]: calculated for C20H24BrN2O5 [M + H+]: 451.08631, found: 451.08619; calculated for C20H2481BrN2O5 [M + H+]: 453.08426, found: 453.08414; RT = 51.9 min (chiral HPLC, chiralpak IC-column and using 30% of mixture of EtOH–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in isohexane as eluent, Flow rate: 0.5 mL min−1) (chiral HPLC, column: chiralpak IC, eluent: 30% of mixture of EtOH–DCM (1
50) in isohexane as eluent, Flow rate: 0.5 mL min−1) (chiral HPLC, column: chiralpak IC, eluent: 30% of mixture of EtOH–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in isohexane, flow rate: 0.5 mL min−1).
50) in isohexane, flow rate: 0.5 mL min−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.41 (dd, J = 8.2, 2.0 Hz, 1H), 7.37* (dd, J = 8.2, 2.0 Hz, 0.28H), 7.34–7.29 (m, 3H), 7.23 (t, J = 2.2 Hz, 1H), 7.18 (d, J = 2.0 Hz, 1H), 7.17* (t, J = 2.2 Hz, 0.28H), 7.16* (d, J = 2.0 Hz, 0.28H), 7.08–7.05 (m, 2H), 7.03* (dd, J = 6.3, 3.2 Hz, 0.56H), 6.51* (d, J = 7.8 Hz, 0.28H), 6.49 (d, J = 8.2 Hz, 1H), 4.96 (d, J = 12.1 Hz, 1H), 4.89 (d, J = 12.1 Hz, 1H), 4.86* (d, J = 12.1 Hz, 0.28H), 4.63 (dd, J = 18.5, 2.2 Hz, 1H), 4.61* (dd, J = 18.5, 2.2 Hz, 0.28H), 4.56 (dd, J = 18.5, 2.2 Hz, 1H), 4.47* (dd, J = 18.3, 2.1 Hz, 0.3H), 2.95* (s, 0.8H), 2.91 (s, 3H), 1.37* (s, 2.6H), 1.09 (s, 9H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 173.56, 173.51*, 160.78*, 160.69, 152.26, 151.86*, 143.90*, 143.78, 141.65*, 141.49, 134.83, 134.06*, 134.00, 132.34, 131.33, 129.00, 128.79, 128.76*, 128.73*, 126.20, 126.03*, 115.17, 115.07*, 110.02*, 109.51, 81.36, 81.31*, 72.30, 67.26, 54.33*, 54.10, 28.53, 28.04, 26.61*, 26.36; HRMS [ESI]: calculated for C25H26BrN2O5 [M + H+]: 513.10196, found: 513.10250; calculated for C25H2681BrN2O5 [M + H+]: 515.09991, found: 515.10048; RT = 17.7 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1
1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.41 (dd, J = 8.2, 2.0 Hz, 1H), 7.37* (dd, J = 8.2, 2.0 Hz, 0.28H), 7.34–7.29 (m, 3H), 7.23 (t, J = 2.2 Hz, 1H), 7.18 (d, J = 2.0 Hz, 1H), 7.17* (t, J = 2.2 Hz, 0.28H), 7.16* (d, J = 2.0 Hz, 0.28H), 7.08–7.05 (m, 2H), 7.03* (dd, J = 6.3, 3.2 Hz, 0.56H), 6.51* (d, J = 7.8 Hz, 0.28H), 6.49 (d, J = 8.2 Hz, 1H), 4.96 (d, J = 12.1 Hz, 1H), 4.89 (d, J = 12.1 Hz, 1H), 4.86* (d, J = 12.1 Hz, 0.28H), 4.63 (dd, J = 18.5, 2.2 Hz, 1H), 4.61* (dd, J = 18.5, 2.2 Hz, 0.28H), 4.56 (dd, J = 18.5, 2.2 Hz, 1H), 4.47* (dd, J = 18.3, 2.1 Hz, 0.3H), 2.95* (s, 0.8H), 2.91 (s, 3H), 1.37* (s, 2.6H), 1.09 (s, 9H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 173.56, 173.51*, 160.78*, 160.69, 152.26, 151.86*, 143.90*, 143.78, 141.65*, 141.49, 134.83, 134.06*, 134.00, 132.34, 131.33, 129.00, 128.79, 128.76*, 128.73*, 126.20, 126.03*, 115.17, 115.07*, 110.02*, 109.51, 81.36, 81.31*, 72.30, 67.26, 54.33*, 54.10, 28.53, 28.04, 26.61*, 26.36; HRMS [ESI]: calculated for C25H26BrN2O5 [M + H+]: 513.10196, found: 513.10250; calculated for C25H2681BrN2O5 [M + H+]: 515.09991, found: 515.10048; RT = 17.7 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in isohexane, flow rate: 0.5 mL min−1).
50) in isohexane, flow rate: 0.5 mL min−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.64 (d, J = 7.1 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.40 (t, J = 7.6 Hz, 1H), 7.30–7.26 (m, 1H), 7.12 (d, J = 7.2 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 6.95* (t, J = 7.4 Hz, 0.15H), 6.85* (d, J = 7.9 Hz, 0.16H), 6.83 (t, J = 2.2 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.75* (t, J = 2.0 Hz, 1H), 4.74 (d, J = 2.1 Hz, 1H), 4.68* (dd, J = 20.5, 2.1 Hz, 1H), 3.35* (s, 0.4H), 3.30 (s, 1H), 1.39* (s, 1.4H), 1.09 (s, 9H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 189.69, 174.38, 152.70, 145.05, 145.01*, 141.60, 140.97, 140.81*, 137.66*, 137.58, 132.97, 132.90*, 129.77*, 129.73, 129.15, 128.66*, 128.63, 122.70, 122.55*, 122.46, 122.33*, 108.68*, 108.20, 81.05, 80.96*, 73.50, 54.95*, 54.71, 28.58, 28.04, 27.05*, 26.76; HRMS [ESI]: calculated for C24H25N2O4 [M + H+]: 405.18088, found: 405.18136; RT = 29.3 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1
1 rotamer ratio, asterisks denote minor rotamer peaks, 400 MHz, CDCl3) δ 7.64 (d, J = 7.1 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.40 (t, J = 7.6 Hz, 1H), 7.30–7.26 (m, 1H), 7.12 (d, J = 7.2 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 6.95* (t, J = 7.4 Hz, 0.15H), 6.85* (d, J = 7.9 Hz, 0.16H), 6.83 (t, J = 2.2 Hz, 1H), 6.81 (d, J = 7.8 Hz, 1H), 6.75* (t, J = 2.0 Hz, 1H), 4.74 (d, J = 2.1 Hz, 1H), 4.68* (dd, J = 20.5, 2.1 Hz, 1H), 3.35* (s, 0.4H), 3.30 (s, 1H), 1.39* (s, 1.4H), 1.09 (s, 9H); 13C NMR (asterisks denote minor rotamer peaks, 101 MHz, CDCl3) δ 189.69, 174.38, 152.70, 145.05, 145.01*, 141.60, 140.97, 140.81*, 137.66*, 137.58, 132.97, 132.90*, 129.77*, 129.73, 129.15, 128.66*, 128.63, 122.70, 122.55*, 122.46, 122.33*, 108.68*, 108.20, 81.05, 80.96*, 73.50, 54.95*, 54.71, 28.58, 28.04, 27.05*, 26.76; HRMS [ESI]: calculated for C24H25N2O4 [M + H+]: 405.18088, found: 405.18136; RT = 29.3 min (chiral HPLC, column: chiralpak IC, eluent: 50% of mixture of EtOH–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in isohexane, flow rate: 0.5 mL min−1).
50) in isohexane, flow rate: 0.5 mL min−1).
Acknowledgements
This research was supported by funds from Max-Planck-Gesellschaft. We thank Nils Schloemp for his help in some experiments.References
- (a) S. Wetzel, R. S. Bon, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 10800–10826 CrossRef CAS; (b) R. S. Bon and H. Waldmann, Acc. Chem. Res., 2010, 43, 1103–1114 CrossRef CAS; (c) K. Grabowski, K. H. Baringhaus and G. Schneider, Nat. Prod. Rep., 2008, 25, 892–904 RSC; (d) A. A. Shelat and R. K. Guy, Nat. Chem. Biol., 2007, 3, 442–446 CrossRef CAS; (e) C. M. Dobson, Nature, 2004, 432, 824–828 CrossRef CAS.
- Selected reviews on spirooxindoles: (a) B. Yu, D. Q. Yu and H. M. Liu, Eur. J. Med. Chem., 2015, 97, 673–698 CrossRef CAS; (b) M. Xia and R. Z. Ma, J. Heterocycl. Chem., 2014, 51, 539–554 CrossRef CAS; (c) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743–762 CrossRef CAS; (d) C. Tsukano and Y. Takemoto, Heterocycles, 2014, 89, 2271–2302 CrossRef CAS; (e) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023–1052 CrossRef CAS; (f) R. Dalpozzo, G. Bartolib and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247–7290 RSC; (g) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS.
- For selected examples of the synthesis of spirooxindoles, see: (a) T. Arai, H. Ogawa, A. Awata, M. Sato, M. Watabe and M. Yamanaka, Angew. Chem., Int. Ed., 2015, 54, 1595–1599 CrossRef CAS; (b) Y. Tian, L. Tian, X. He, C. Li, X. Jia and J. Li, Org. Lett., 2015, 17, 4874–4877 CrossRef CAS; (c) W. Dai, X.-L. Jiang, Q. Wu, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 5737–5744 CrossRef CAS; (d) G. O. Fonseca, Z.-J. Wang, O. A. Namjoshi, J. R. Deschamps and J. M. Cook, Tetrahedron Lett., 2015, 56, 3052–3056 CrossRef CAS; (e) D. Zhang, D.-M. Zhang, G.-Y. Xu and J.-T. Sun, Chin. Chem. Lett., 2015, 26, 301–303 CrossRef CAS; (f) K. Suman, L. Srinu and S. Thennarasu, Org. Lett., 2014, 16, 3732–3735 CrossRef CAS; (g) F. Salahi, M. J. Taghizadeh, H. Arvinnezhad, M. Moemeni, K. Jadidi and B. Notash, Tetrahedron Lett., 2014, 55, 1515–1518 CrossRef CAS; (h) K. O. Takasuke Mukaiyama, I. Sato and Y. Hayashi, Chem.–Eur. J., 2014, 20, 13583–13588 CrossRef; (i) L. Wang, X.-M. Shi, W.-P. Dong, L.-P. Zhu and R. Wang, Chem. Commun., 2013, 49, 3458–3460 RSC; (j) W. Tan, X.-T. Zhu, S. Zhang, G.-J. Xing, R.-Y. Zhu and F. Shi, RSC Adv., 2013, 3, 10875–10886 RSC; (k) S. N. Singh, S. Regati, A. K. Paul, M. Layek, S. Jayaprakash, K. V. Reddy, G. S. Deora, S. Mukherjee and M. Pal, Tetrahedron Lett., 2013, 54, 5448–5452 CrossRef CAS; (l) A. Dandia, A. K. Jain and A. K. Laxkar, Tetrahedron Lett., 2013, 54, 3929–3932 CrossRef CAS; (m) W.-T. Wei, C.-X. Chen, R.-J. Lu, J.-J. Wang, X.-J. Zhang and M. Yan, Org. Biomol. Chem., 2012, 10, 5245–5252 RSC; (n) G. Bhaskar, Y. Arun, C. Balachandran, C. Saikumar and P. T. Perumal, Eur. J. Med. Chem., 2012, 51, 79–91 CrossRef CAS; (o) B. Tan, X. Zeng, W. W. Y. Leong, Z. Shi, C. F. Barbas III and G. Zhong, Chem.–Eur. J., 2012, 18, 63–67 CrossRef CAS; (p) Y. Cao, X. Jiang, L. Liu, F. Shen, F. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 9124–9127 CrossRef CAS; (q) A. P. Antonchick, H. Schuster, H. Bruss, M. Schürmann, H. Preut, D. Rauh and H. Waldmann, Tetrahedron, 2011, 67, 10195–10202 CrossRef CAS; (r) H. Chen, S.-Y. Wang, X.-P. Xu and S.-J. Ji, Synth. Commun., 2011, 41, 3280–3288 CrossRef CAS; (s) R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, Eur. J. Med. Chem., 2009, 44, 3821–3829 CrossRef; (t) A. V. Babenysheva and A. N. Maslivets, Russ. J. Org. Chem., 2008, 44, 1401–1402 CrossRef CAS.
- (a) K.-K. Wang, T. Jin, X. Huang, Q. Ouyang, W. Du and Y.-C. Chen, Org. Lett., 2016, 18, 872–875 CrossRef CAS; (b) P. Cheng, W. Guo, P. Chen, Y. Liu, X. Du and C. Li, Chem. Commun., 2016, 52, 3418–3421 RSC; (c) Y. Shi, A. Lin, H. Mao, Z. Mao, W. Li, H. Hu, C. Zhu and Y. Cheng, Chem.–Eur. J., 2013, 19, 1914–1918 CrossRef CAS; (d) G. Wang, X. Liu, T. Huang, Y. Kuang, L. Lin and X. Feng, Org. Lett., 2013, 15, 76–79 CrossRef CAS; (e) L. Hong, M. Kai, C. Y. Wu, W. S. Sun, G. M. Zhu, G. F. Li, X. J. Yao and R. Wang, Chem. Commun., 2013, 49, 6713–6715 RSC; (f) W. S. Sun, G. M. Zhu, C. Y. Wu, G. F. Li, L. Hong and R. Wang, Angew. Chem., Int. Ed., 2013, 52, 8633–8637 CrossRef CAS; (g) H. L. Zhang, L. Hong, H. Kang and R. Wang, J. Am. Chem. Soc., 2013, 135, 14098–14101 CrossRef CAS; (h) H. L. Zhang, X. Z. Ma, H. Kang, L. Hong and R. Wang, Chem.–Asian J., 2013, 8, 542–545 CrossRef CAS; (i) N. V. Hanhan, N. R. Ball-Jones, N. T. Tran and A. K. Franz, Angew. Chem., 2012, 124, 1013–1016 CrossRef; (j) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837–7841 CrossRef CAS; (k) B. Tan, N. R. Candeias and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 4672–4675 CrossRef CAS; (l) K. Selvakumar, V. Vaithiyanathan and P. Shanmugam, Chem. Commun., 2010, 46, 2826–2828 RSC; (m) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schurmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735–740 CrossRef CAS; (n) X. H. Chen, Q. Wei, S. W. Luo, H. Xiao and L. Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819–13825 CrossRef CAS; (o) M. P. Castaldi, D. M. Troast and J. A. Porco, Org. Lett., 2009, 11, 3362–3365 CrossRef CAS; (p) M. M. C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein and S. L. Schreiber, J. Am. Chem. Soc., 2004, 126, 16077–16086 CrossRef CAS; (q) P. R. Sebahar and R. M. Williams, J. Am. Chem. Soc., 2000, 122, 5666–5667 CrossRef CAS.
- For reviews on phosphine catalysis, see: (a) Y. Xiao, H. Guo and O. Kwon, Aldrichimica Acta, 2016, 49, 3–13 CAS; (b) Z. Wang, X. Xub and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927–2940 RSC; (c) Y. M. Xiao, Z. H. Sun, H. C. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089–2121 CrossRef; (d) Q. Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724–1732 RSC; (e) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005–1018 CrossRef CAS; (f) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102–3116 RSC.
- (a) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587–4591 CrossRef CAS; (b) C. E. Henry, Q. Xu, Y. C. Fan, T. J. Martin, L. Belding, T. Dudding and O. Kwon, J. Am. Chem. Soc., 2014, 136, 11890–11893 CrossRef CAS; (c) Y. Wei and M. Shi, Chem.–Asian J., 2014, 9, 2720–2734 CrossRef CAS; (d) H. Yu, L. Zhang, Z. Yang, Z. Li, Y. Zhao, Y. Xiao and H. Guo, J. Org. Chem., 2013, 78, 8427–8436 CrossRef CAS; (e) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767–770 CrossRef CAS; (f) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764–2766 RSC; (g) H. Xiao, Z. Chai, H.-F. Wang, X.-W. Wang, D.-D. Cao, W. Liu, Y.-P. Lu, Y.-Q. Yang and G. Zhao, Chem.–Eur. J., 2011, 17, 10562–10565 CrossRef CAS; (h) N. Pinto, P. Retailleau, A. Voituriez and A. Marinetti, Chem. Commun., 2011, 47, 1015–1017 RSC; (i) M. Schuler, A. Voituriez and A. Marinetti, Tetrahedron: Asymmetry, 2010, 21, 1569–1573 CrossRef CAS; (j) N. Pinto, M. Neel, A. Panossian, P. Retailleau, G. Frison, A. Voituriez and A. Marinetti, Chem.–Eur. J., 2010, 16, 1033–1045 CrossRef CAS; (k) A. Voituriez, N. Pinto, M. Neel, P. Retailleau and A. Marinetti, Chem.–Eur. J., 2010, 16, 12541–12544 CrossRef CAS; (l) N. Pinto, N. Fleury-Brégeot and A. Marinetti, Eur. J. Org. Chem., 2009, 146–151 CAS; (m) Y. Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660–5661 CrossRef CAS; (n) N. Fleury-Brégeot, L. Jean, P. Retailleau and A. Marinetti, Tetrahedron, 2007, 63, 11920–11927 CrossRef; (o) L. Jean and A. Marinetti, Tetrahedron Lett., 2006, 47, 2141–2145 CrossRef CAS; (p) A. Scherer and J. A. Gladysz, Tetrahedron Lett., 2006, 47, 6335–6337 CrossRef CAS; (q) R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234–12235 CrossRef CAS.
- (a) P. Zheng, C. A. Gondo and J. W. Bode, Chem.–Asian J., 2011, 6, 614–620 CrossRef CAS; (b) B. M. Trost and S. M. Silverman, J. Am. Chem. Soc., 2010, 132, 8238–8240 CrossRef CAS.
- (a) M. Holmquist, G. Blay and J. R. Pedro, Chem. Commun., 2014, 50, 9309–9312 RSC; (b) M. G. Sankar, M. Garcia-Castro, Y. Wang and K. Kumar, Asian J. Org. Chem., 2013, 2, 646–649 CrossRef CAS; (c) W. Yan, D. Wang, J. Feng, P. Li, D. Zhao and R. Wang, Org. Lett., 2012, 14, 2512–2515 CrossRef CAS.
- H. Lv, B. Tiwari, J. Mo, C. Xing and Y. R. Chi, Org. Lett., 2012, 14, 5412–5415 CrossRef CAS.
- H.-M. Zhang, Z.-H. Gao and S. Ye, Org. Lett., 2014, 16, 3079–3081 CrossRef CAS.
- W.-Q. Jia, H.-M. Zhang, C.-L. Zhang, Z.-H. Gao and S. Ye, Org. Chem. Front., 2016, 3, 77–81 RSC.
- K. Zhao, Y. Zhi, X. Li, R. Puttreddy, K. Rissanen and D. Enders, Chem. Commun., 2016, 52, 2249–2252 RSC.
- P. Cheng, W. Guo, P. Chen, Y. Liu, X. Du and C. Li, Chem. Commun., 2016, 52, 3418–3421 RSC.
- While the manuscript was in preparation, Han, Lu and co-workers reported an asymmetric [3 + 2]-annulation reaction between allene esters and isatinimines catalyzed by L-threonine derived aminophosphine: X. Han, W.-L. Chan, W. Yao, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492–6496 CrossRef CAS.
- (a) D. Wang, G.-P. Wang, Y.-L. Sun, S.-F. Zhu, Y. Wei, Q.-L. Zhou and M. Shi, Chem. Sci., 2015, 6, 7319–7325 RSC; (b) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764–2766 RSC.
- (a) A. Danda, N. Kesava-Reddy, C. Golz, C. Strohmann and K. Kumar, Org. Lett., 2016, 18, 2632–2635 CrossRef CAS; (b) W. Li and J. Zhang, Chem. Soc. Rev., 2016, 45, 1657–1677 RSC; (c) P. Y. Dakas, J. A. Parga, S. Hoing, H. R. Scholer, J. Sterneckert, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2013, 52, 9576–9581 CrossRef CAS; (d) H. Xiao, Z. Chai, D. D. Cao, H. Y. Wang, J. H. Chen and G. Zhao, Org. Biomol. Chem., 2012, 10, 3195–3201 RSC; (e) H. Xiao, Z. Chai, C. W. Zheng, Y. Q. Yang, W. Liu, J. K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467–4470 CrossRef CAS.
- CCDC 1472155† contains the supplementary X-ray crystallographic data for compound 3j and can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- (a) K. Zhang, L. C. Cai, X. Jiang, M. A. Garcia-Garibay and O. Kwon, J. Am. Chem. Soc., 2015, 137, 11258–11261 CrossRef CAS; (b) Z. Lu, S. Zheng, X. Zhang and X. Lu, Org. Lett., 2008, 10, 3267–3270 CrossRef CAS; (c) K. Kumar, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 787–789 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1472155. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra12387b |
| This journal is © The Royal Society of Chemistry 2016 |