Open Access Article
Open Access ArticleCuCl/air-mediated oxidative coupling reaction of imidazoheterocycles with N-aryl glycine esters†
Jing
Jiao
ab,
Jun-Rong
Zhang
a,
Yan-Yan
Liao
a,
Li
Xu
a,
Maolin
Hu
*b and
Ri-Yuan
Tang
 *ab
*ab
aDepartment of Applied Chemistry, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China. E-mail: rytang@scau.edu.com
bCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, China. E-mail: maolin@wzu.edu.cn; Tel: +86-577-8668-9615
First published on 12th June 2017
Abstract
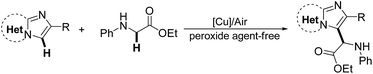
A copper/air mediated oxidative coupling reaction of imidazoheterocycles with N-aryl glycine esters is here described. The reaction proceeded effectively under an air atmosphere without the use of peroxide agents. This simple protocol allows for the preparation of a wide range of imidazoheterocycles with a glycine ester motif, which are of great interest in the field of medicinal chemistry. Interestingly, the coupling of imidazo[1,2-a]pyridine with secondary or tertiary α-amino phenylethanone selectively affords the imidazo[1,2-a]pyridin-3-yl imine or imidazo[1,2-a]pyridin-3-yl diketone in the presence of CuCl and TBHP (tertbutyl hydroperoxide).
Introduction
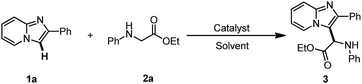
Glycine is a major protein component and one of the most important natural amino acids in living organisms. It has a wide range of biological and physiological properties.1 Glycine esters are particularly useful building blocks for the construction of a variety of biological molecules.2 We envisioned that the coupling of glycine ester with imidazoheterocycles can produce intriguing molecules for drug screening because imidazoheterocycles are promising drug skeletons with a broad spectrum of biological activity, including antitumor, antiviral, antifungal, and antibacterial activity.3,4 Considerable effort has been devoted to the introduction of many different pharmacophores (such as thiol,5 thiocyano,6 dithiocarbamyl,7 phosphonyl,8 trifluoromethyl,9 trifluoromethylthiol,10 and esters11) onto imidazoheterocycles. The coupling of imidazoheterocycles to glycine ester not only affords useful biological applications but also contributes to synthetic chemistry. However, the coupling of imidazole heterocycles to glycine esters has not yet been studied.The C–H oxidative cross-coupling strategy is an ideal route for the coupling of imidazole heterocycles with glycine esters. In recent years, there has been more interest in the study of the oxidative cross-coupling reaction in organic chemistry.12 Increasing attention has been paid to the oxidative C–H functionalization at the α-position of N-arylglycine ester.13,14 The oxidative cross-coupling of glycine esters under oxidant-free conditions and the use of oxygen rather than stoichiometric peroxide agent is desirable.14 For example, in 2014, Huo and coauthors reported an auto-oxidative cross-coupling reaction of glycine derivatives and short peptides in a simple mixed organic solvent under mild reaction conditions.14c Wu et al. reported a visible light catalysis-assisted coupling reaction of α-amino acids with β-keto esters or indole derivatives in the absence of oxidant.14b Huang et al. developed a novel cross-coupling reaction between methylquinoline derivatives and N-arylglycine esters by a cooperative catalysis of copper salt and Brønsted acid.14d It has been demonstrated that imidazoheterocycles can capture a trifluoromethyl free radical initiated by TBHP.9 It is here reasoned that the coupling of imidazoheterocycles to glycine esters may be carried out via an oxidative free radical process. However, imidazopyridines may encounter an oxidative ring opening in the presence of TBHP at 120 °C.15 In this way, the development of a TBHP-free protocol for the coupling of imidazoheterocycles with glycine esters under mild conditions is desirable. Herein, we report a copper/air-mediated oxidative coupling of imidazoheterocycles to glycine esters in the absence of peroxide agent (Scheme 1).
Results and discussion
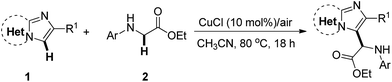
The current investigation began with the coupling of imidazopyridine 1a with glycine ester 2a for the optimization of reaction conditions (Table 1). Several copper catalysts such as CuI, CuBr, CuCl, and Cu(OAc)2 were evaluated in CH3CN at 80 °C (entries 1–4). The reactivity sequence of copper halides is CuCl > CuBr > CuI. The reaction with 10 mol% of CuCl proceeded well in air, giving product 3 in 78% yield (entry 3). Unexpectedly, Cu(OAc)2 did not work and no product 3 was observed, though some of substrate 2a was converted into ethyl 2-oxo-2-(phenylamino)acetate (entry 4). Next, several other solvents including EtOH, DMF, 1,4-dioxane, and toluene were examined. All of these solvents also worked, but were less effective than CH3CN (entries 5–9). It was shown that the reduction of CuCl loading to 5 mol% led to a slight decrease in the yield of product 3 (76% yield, entry 10). The reaction with 1 mol% of CuCl was still carried out to give product 3 in 56% yield (entry 11). In the absence of CuCl, the reaction could not be carried out (entry 12). The results showed that either decreasing or elevating the temperature led to a decrease in the yield to some extent, e.g. 52% yield at 60 °C and 67% yield at 100 °C (entries 13 and 14). The reaction could not take place in an N2 atmosphere, suggesting that the O2 in the air is crucial to the transformation (entry 15). Encouraged by this result, an attempt was made to increase the product yield of the reaction that took place under O2. However, the product yield was not increased (entry 16). These results suggest that the O2 in the air is sufficient to induce the transformation. Next, several oxidants such as TBHP, H2O2, K2S2O8, and oxone (2KHSO5·KHSO4·K2SO4) were investigated with 10 mol% of CuCl in CH3CN at 80 °C (entries 17–20). The reaction with TBHP and CuCl gave product 3 in 78% yield (entry 17). Other oxidants inhibited this reaction, leading to a decrease in the yield or inhibition of the reaction entirely (entries 18–20).| Entry | [Cu] | Oxidant | Solvent | T (°C) | Isolated yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), [Cu] (10 mol%), oxidant (2 equiv.), solvent (2 mL), reaction for 18 h under air atmosphere. b CuCl (5 mol%). c CuCl (1 mol%). d Under N2 (1 atm). e Under O2 (1 atm). f TBHP (5.5 mol L−1 in decane). | |||||
| 1 | CuI | — | CH3CN | 80 | 40 |
| 2 | CuBr | — | CH3CN | 80 | 51 |
| 3 | CuCl | — | CH3CN | 80 | 78 |
| 4 | Cu(OAc)2 | — | CH3CN | 80 | 0 |
| 5 | CuCl | — | DCE | 80 | 65 |
| 6 | CuCl | — | EtOH | 80 | 46 |
| 7 | CuCl | — | DMF | 80 | 43 |
| 8 | CuCl | — | 1,4-Dioxane | 80 | 8 |
| 9 | CuCl | — | Toluene | 80 | 24 |
| 10b | CuCl | — | CH3CN | 80 | 76 |
| 11c | CuCl | — | CH3CN | 80 | 56 |
| 12 | — | — | CH3CN | 80 | 0 |
| 13 | CuCl | — | CH3CN | 60 | 52 |
| 14 | CuCl | — | CH3CN | 100 | 67 |
| 15d | CuCl | — | CH3CN | 80 | NR |
| 16e | CuCl | O2 | CH3CN | 80 | 75 |
| 17f | CuCl | TBHP | CH3CN | 80 | 78 |
| 18 | CuCl | H2O2 | CH3CN | 80 | 0 |
| 19 | CuCl | K2S2O8 | CH3CN | 80 | Trace |
| 20 | CuCl | Oxone | CH3CN | 80 | 40 |
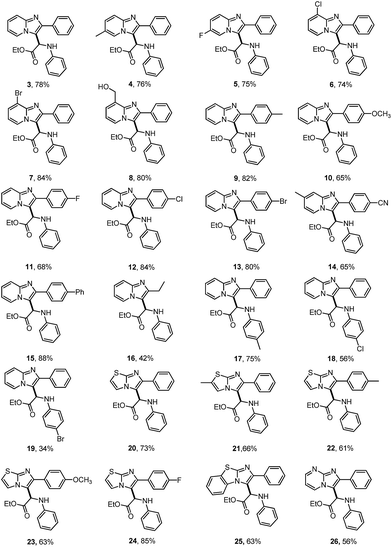
The scope for the reaction of imidazoheterocycles with N-arylglycine esters 2 was investigated with these optimized reaction conditions (Table 1, entry 3; Table 2). Results showed that the current oxidative coupling reaction was suitable to a wide range of imidazopyridines and imidazothiazoles (products 3–26). The synthetically valuable functional groups on the pyridine ring (such as halo and methoxy groups) were well tolerated. For example, the substrate with a bromo group on the pyridine ring underwent the reaction smoothly and produced the desired product 7 at a yield of 84%. The hydroxyl group turned out to have strong tolerance under standard conditions, producing product 8 at a yield of 80%.
| a Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol, 1.5 equiv.), CuCl (10 mol%), and CH3CN (2 mL) at 80 °C for 18 h under air. |
|---|
|
Next, the effect of various substituents on the benzene ring of imidazoheterocycles was examined. Both electron-donating and electron-withdrawing groups were well tolerated, producing the corresponding products in synthetically useful yields (65–84% yield, products 9–14). The reaction proceeded well with a diphenyl substituted imidazopyridine, giving product 15 at a yield of 88%. The ethyl substituted imidazopyridine has poorer reactivity than these aryl groups, giving product 16 at a yield of 42%. Subsequently, N-arylglycine esters with a substituent (for example, methyl, chloro, or bromo) on the benzene ring were also examined (products 17–19). Both chloro and bromo groups reduced the reactivity of N-phenyl glycine esters, leading to poor yields (products 18 and 19). Encouragingly, imidazothiazoles were also compatible with the standard conditions (product 20–25). For example, the reaction of 2-phenylimidazothiazoles with a methoxy or fluoro group on the benzene ring proceeded well to produce the corresponding products 23 and 24 in 63% and 85% yields, respectively. The fused benzo[d]imidazo[2,1-b]thiazole also performed well, producing product 25 at a yield of 63%. 2-Phenylimidazo[1,2-a]pyrimidine was also found to be a suitable substrate for this transformation, affording product 26 at a yield of 56%. However, the reaction did not proceed with N-Boc, N-Bz or N-methyl protected glycine esters under the reaction conditions. N-Unprotected glycine ester and glycine were not suitable substrates for this reaction. These substrates may not be oxidized to the corresponding imine cation for the nucleophilic addition with imidazoheterocycles (Scheme 5). Unfortunately, the reaction of N-phenyl alanine ester did not occur, which may result from the steric hindrance of the tertiary carbon center.
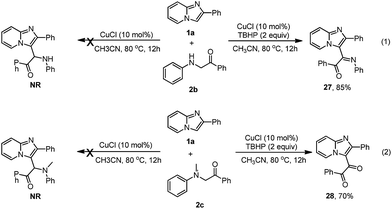
The coupling reaction between α-amino phenylethanone and imidazopyridine 1a was also investigated. As shown in Scheme 2, both 1-phenyl-2-(phenylamino)ethanone 2b and 2-(methyl(phenyl)amino)-1-phenylethanone 2c were unable to react with imidazopyridine 1a to produce the desired product under the standard conditions. Imidazopyridine 1a was quantitatively recovered, and most of substrate 2b (or 2c) was decomposed by the C–N bond cleavage and was oxidized to the corresponding 2-oxo-acetamide. Interestingly, the reaction of 1a with 2b was able to take place in the presence of TBHP and selectively afforded a 1-phenyl-2-(2-phenylimidazo[1,2-a]pyridin-3-yl)-2-(phenylimino)ethanone 27 in 85% yield (eqn (1)). Replacing 2b with tertiary amine 2c caused the coupling reaction of 1a to 2c to selectively produce a diketone product 28 in 70% yield (eqn (2)).16 These results are similar to those obtained from the coupling of indole to 2b reported by Li et al.17
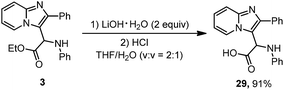
Imidazoheterocycles with glycine motifs are of considerable interest to researchers and may have applications in biochemistry. Compound 3 was hydrolyzed to amino acid 29 (Scheme 3). The hydrolysis proceeded smoothly in the presence of LiOH at 0 °C. After treatment with HCl aqueous solution, a 91% yield of product 29 was obtained.
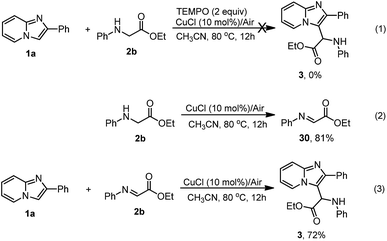
To better understand the reaction pathway, several control experiments were conducted as shown in Scheme 4. The reaction could not take place in the presence of two equivalents of TEMPO (2,2,6,6-tetramethylpiperidinooxy, a free radical trapping agent), suggesting that this reaction may undergo a radical process (Scheme 4, eqn (1)). Under standard conditions, N-phenyl glycine ester was oxidized to ethyl 2-(phenylimino)acetate at a yield of 81% (Scheme 4, eqn (2)). Interestingly, imidazopyridine 1a underwent a Friedel–Crafts reaction with ethyl 2-(phenylimino)acetate (30) to produce the desired product 3 at a yield of 72% (Scheme 4, eqn (3)). The results demonstrated that transformation of N-phenyl glycine ester into certain active species was crucial to the coupling reaction.
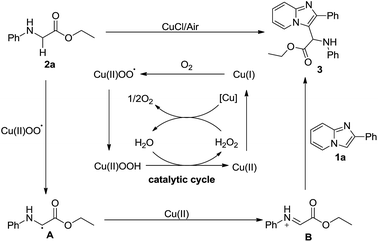
Based on the results of previous reports,14 a plausible mechanism was proposed and is outlined in Scheme 5. Initially, the oxygen reacts with Cu(I) to produce Cu(II) peroxide radicals, which might abstract an α-hydrogen atom of glycine ester 2a to form radical A, followed by an oxidation with Cu(II) to form imine cation B. Subsequently, imidazopyridine 1a undergoes the Friedel–Crafts reaction with imine cation B to produce the desired product 3.14e,18
Conclusions
In summary, a novel CuCl/air-mediated oxidative cross-coupling of imidazoheterocycles with glycine esters was here developed for the synthesis of imidazoheterocycles with glycine ester motifs. These novel compounds are of great interest in medicinal chemistry. This copper-catalyzed coupling reaction can be carried out in air. Interestingly, in the presence of TBHP and CuCl, the coupling reaction of imidazopyridine 1a with secondary α-aminophenylethanone selectively produced imidazo[1,2-a]pyridin-3-yl imine 27, whereas the reaction with tertiary α-aminophenylethanone selectively provided imidazo[1,2-a]pyridin-3-yl diketone 28 at a good yield.Experimental section
General remarks
1H and 13C NMR spectra were measured on a Bruker Avance-III 500 instrument (500 MHz for 1H, 125 MHz for 13C NMR spectroscopy) using CDCl3 or DMSO-d6 as the solvent. Chemical shifts for 1H and 13C NMR were evaluated by reference to internal Me4Si (0 ppm) as the standard. The following abbreviations (and combinations thereof) are here used to designate multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Mass spectra were measured on a Shimadzu GC-MS-QP2010 Plus spectrometer (EI). HRMS (ESI) analysis was performed on a Bruker micrOTOF-Q II instrument. IR analysis was measured on a Nicolet IS10 spectrometer (ATR).General procedure for the oxidative coupling reaction of imidazoheterocycles with glycine esters
A 15 mL tube with a Teflon cap, equipped with a magnetic stirring bar, was charged with substrate 1a (0.20 mmol), N-phenyl glycine ester 2a (0.30 mmol), CuCl (10 mol%) in air, CH3CN (2 mL) was then added sequentially. The tube was then capped and stirred at 80 °C for 18 h, the crude mixture was diluted with AcOEt, and washed with saturated brine. The organic phase was collected and dried over Na2SO4, filtered through a Celite pad, and washed with AcOEt. The filtrate was concentrated in vacuo, and the resulting residue was purified using column chromatography (petroleum–EtOAc) to produce product 3 as a yellow solid.Experimental procedure for the hydrolysis of compound 3
A 50 mL round-bottom flask equipped with a magnetic stirring bar was charged with compound 3 (2.0 mmol) and LiOH·H2O (2.0 equiv.), THF (10 mL), and water (5 mL) were then added sequentially at 0 °C. The reaction mixture was stirred at 0 °C for 2 h. Next, the reaction mixture was acidified (pH = 3–4) using HCl aq. solution (2 N). The precipitate was filtered and dried to afford product 29 as a white solid.Typical procedure for the oxidative coupling reaction of imidazopyridine 1a with α-amino phenylethanone 2b or 2c
A 15 mL tube with a Teflon cap, equipped with a magnetic stirring bar, was charged with substrate 1a (0.20 mmol), α-amino phenylethanone 2b (or 2c for product 28) (0.30 mmol), CuCl (10 mol%), CH3CN (2 mL) in air, TBHP (5.5 mol L−1 in decane, 2 equiv.) was then added sequentially. The tube was then capped and stirred at 80 °C for 12 h. The crude mixture was diluted with AcOEt and washed with saturated brine. The organic phase was collected and dried over Na2SO4, filtered through a Celite pad, and washed with AcOEt. The filtrate was concentrated in vacuo, and the resulting residue was purified using column chromatography (petroleum–EtOAc) to produce product 27 as a yellow solid.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21202121, 21371137 and 21571143) and Guangzhou Science Technology Program Project (No. 201607010142) for their financial support.Notes and references
- (a) G. E. Barrett and D. T. Elmore, Amino Acids and Peptides, Cambridge University, Cambridge, 1998 CrossRef; (b) M. Calmes and J. Daunis, Amino Acids, 1999, 16, 215 CrossRef CAS.
- (a) K. Maruoka and T. Ooi, Chem. Rev., 2003, 103, 3013 CrossRef CAS PubMed; (b) M. Castanho and N. Santos, Peptide Drug Discovery and Development: Translational Research in Academia and Industry, Wiley-VCH, Weinheim, 2011 CrossRef; (c) S. Baktharaman, R. Hili and A. K. Yudin, Aldrichimica Acta, 2008, 41, 109 CAS; (d) R. L. Polt, in Amino Acid Derivatives: A Practical Approach, ed. G. C. Barrett, Oxford University Press, Oxford, UK, 1999, pp. 101–114 Search PubMed; (e) F. D. Klingler, Acc. Chem. Res., 2007, 40, 1367 CrossRef CAS J. W. Grate and G. C. Frye, in Sensors Update, ed. H. Baltes, W. Göpel and J. Hesse, Wiley-VCH, Weinheim, 1996, vol. 2, pp. 10–20 Search PubMed.
- (a) C. Enguehard-Gueiffier and A. Gueiffier, Mini-Rev. Med. Chem., 2007, 7, 888 CrossRef CAS; (b) F. Couty and G. Evano, in Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier Science, Oxford, 2008, vol. 11, pp. 409–492, and references therein Search PubMed.
- (a) A. Andreani, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L. Varoli, N. Calonghi, C. Cappadone, G. Farruggia, M. Zini, C. Stefanelli, L. Masotti, N. S. Radin and R. H. Shoemaker, J. Med. Chem., 2008, 51, 809 CrossRef CAS; (b) J.-H. Park, M. I. El-Gamal, Y. S. Lee and C.-H. Oh, Eur. J. Med. Chem., 2011, 46, 5769 CrossRef CAS; (c) A. R. Ali, E. R. El-Bendary, M. A. Ghaly and I. A. Shehata, Eur. J. Med. Chem., 2014, 75, 492 CrossRef CAS.
- (a) C. Ravi, D. Chandra Mohan and S. Adimurthy, Org. Biomol. Chem., 2016, 14, 2282 RSC; (b) Y. Ding, W. Wu, W. Zhao, Y. Li, P. Xie, Y. Huang, Y. Liu and A. Zhou, Org. Biomol. Chem., 2016, 14, 1428 RSC; (c) A. K. Bagdi, S. Mitra, M. Ghosh and A. Hajra, Org. Biomol. Chem., 2015, 13, 3314 RSC; (d) X.-M. Ji, S.-J. Zhou, F. Chen, X.-G. Zhang and R.-Y. Tang, Synthesis, 2015, 47, 659 CrossRef CAS; (e) M.-A. Hiebel and S. Berteina-Raboin, Green Chem., 2015, 17, 937 RSC; (f) C. Ravi, D. Chandra Mohan and S. Adimurthy, Org. Lett., 2014, 16, 2978 CrossRef CAS.
- (a) D. Yang, K. Yan, W. Wei, G. Li, S. Lu, C. Zhao, L. Tian and H. Wang, J. Org. Chem., 2015, 80, 11073 CrossRef CAS; (b) S. Mitra, M. Ghosh, S. Mishra and A. Hajra, J. Org. Chem., 2015, 80, 8275 CrossRef CAS.
- J. Jiao, L. Wei, X.-M. Ji, M.-L. Hu and R.-Y. Tang, Adv. Synth. Catal., 2016, 358, 268 CrossRef CAS.
- M. Yadav, S. Dara, V. Saikam, M. Kumar, S. K. Aithagani, S. Paul, R. A. Vishwakarma and P. P. Singh, Eur. J. Org. Chem., 2015, 2015, 6526 CrossRef CAS.
- (a) K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, J. Org. Chem., 2015, 80, 1332 CrossRef CAS; (b) X.-M. Ji, L. Wei, F. Chen and R.-Y. Tang, RSC Adv., 2015, 5, 29766 RSC.
- Q. Wang, Z. Qi, F. Xie and X. Li, Adv. Synth. Catal., 2015, 357, 355 CrossRef CAS.
- (a) S. Wang, X. Huang, Z. Ge, X. Wang and R. Li, RSC Adv., 2016, 6, 63532 RSC; (b) S. Mishra, P. Mondal, M. Ghosh, S. Mondal and A. Hajra, Org. Biomol. Chem., 2016, 14, 1432 RSC; (c) K. Li, X.-M. Zhao, F.-L. Yang, X.-H. Hou, Y. Xu, Y.-C. Guo, X.-Q. Hao and M.-P. Song, RSC Adv., 2015, 5, 90478 RSC.
- (a) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS; (b) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS; (c) M. Klussmann and D. Sureshkumar, Synthesis, 2011, 353 CrossRef CAS; (d) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS.
- (a) Z.-Q. Zhu and Z.-Z. Huang, Org. Lett., 2016, 18, 1526 CrossRef; (b) K. Li, Q. Wu, J. Lan and J. You, Nat. Commun., 2015, 6, 8404 CrossRef CAS; (c) H. Peng, J.-T. Yu, Y. Jiang, H. Yang and J. Cheng, J. Org. Chem., 2014, 79, 9847 CrossRef CAS; (d) W.-T. Wei, R.-J. Song and J.-H. Li, Adv. Synth. Catal., 2014, 356, 1703 CrossRef CAS; (e) J. Xie and Z.-Z. Huang, Angew. Chem., Int. Ed., 2010, 49, 10181 CrossRef CAS; (f) L. Zhao and C.-J. Li, Angew. Chem., Int. Ed., 2008, 47, 7075 CrossRef CAS; (g) G. Zhang, Y. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 10429 CrossRef CAS; (h) Y. Zhang, M. Nia and B. Feng, Org. Biomol. Chem., 2016, 14, 1550 RSC; (i) L. Zhao, O. Baslé and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4106 CrossRef CAS.
- (a) Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045 CrossRef CAS; (b) X.-W. Gao, Q.-Y. Meng, J.-X. Li, J.-J. Zhong, T. Lei, X.-B. Li, C.-H. Tung and L.-Z. Wu, ACS Catal., 2015, 5, 2391 CrossRef CAS; (c) C. Huo, Y. Yuan, M. Wu, X. Jia, X. Wang, F. Chen and J. Tang, Angew. Chem., Int. Ed., 2014, 53, 13544 CrossRef CAS PubMed; (d) Z.-Q. Zhu, P. Bai and Z.-Z. Huang, Org. Lett., 2014, 16, 4881 CrossRef CAS; (e) C. Huo, C. Wang, M. Wu, X. Jia, H. Xie and Y. Yuan, Adv. Synth. Catal., 2014, 356, 411 CrossRef CAS; (f) X.-W. Gao, Q.-Y. Meng, M. Xiang, B. Chen, K. Feng, C.-H. Tung and L.-Z. Wu, Adv. Synth. Catal., 2013, 355, 2158 CrossRef CAS; (g) S. Zhu and M. Rueping, Chem. Commun., 2012, 48, 11960 RSC; (h) Z.-Q. Wang, M. Hu, X.-C. Huang, L.-B. Gong, Y.-X. Xie and J.-H. Li, J. Org. Chem., 2012, 77, 8705 CrossRef CAS.
- K. Yan, D. Yang, W. Wei, G. Li, M. Sun, Q. Zhang, L. Tian and H. Wang, RSC Adv., 2015, 5, 100102 RSC.
- R.-Y. Tang, X.-K. Guo, J.-N. Xiang and J.-H. Li, J. Org. Chem., 2013, 78, 11163 CrossRef CAS.
- J.-C. Wu, R.-J. Song, Z.-Q. Wang, X.-C. Huang, Y.-X. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2012, 51, 3453 CrossRef CAS.
- G. Desimoni, G. Faita, M. Mella, M. Toscanini and M. Boiocchi, Eur. J. Org. Chem., 2008, 6232 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C spectra for all compounds. See DOI: 10.1039/c7ra05303g |
| This journal is © The Royal Society of Chemistry 2017 |