Themed collection Total synthesis in OBC

Total synthesis of bioactive tetracyclic norditerpene dilactones
Tetracyclic norditerpene dilactones are an important class of terpenoids that have been isolated from both terrestrial and marine sources, typically from Podocarpus plants and from filamentous fungi.
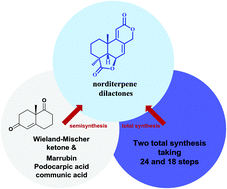
Org. Biomol. Chem., 2021,19, 9138-9147
https://doi.org/10.1039/D1OB01535D
Total synthesis of the pseudoindoxyl class of natural products
In this review, we highlight the completed as well as the formal total synthesis of the spiro-pseudoindoxyl natural products, with a focus on methodologies developed for this key structural unit, challenges associated at the strategy level and the a future perspective.
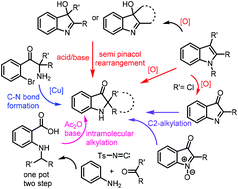
Org. Biomol. Chem., 2021,19, 7970-7994
https://doi.org/10.1039/D1OB01285A
The biology and total syntheses of bisbenzylisoquinoline alkaloids
This mini-review provides a concise overview of the biosynthetic pathway and pharmacology of bisbenzylisoquinoline alkaloid (bisBIA) natural products.
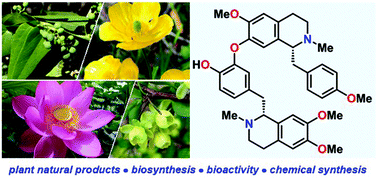
Org. Biomol. Chem., 2021,19, 7535-7543
https://doi.org/10.1039/D1OB00812A
Recent reports on the synthesis of γ-butenolide, γ-alkylidenebutenolide frameworks, and related natural products
This review focusses on synthetic strategies for γ-butenolides from 2010 to 2020 with an emphasis on γ-alkylidenebutenolides and related molecules. Metal-mediated catalytic transformation and organocatalysis are the two main reaction partners that were widely used.
Org. Biomol. Chem., 2021,19, 7298-7332
https://doi.org/10.1039/D1OB00875G
Contemporary advancements in the semi-synthesis of bioactive terpenoids and steroids
This review discusses the reemergence of semi-synthesis as an efficient approach to prepare bioactive complex terpenoids and steroids.

Org. Biomol. Chem., 2021,19, 3791-3812
https://doi.org/10.1039/D1OB00448D
Nonbiomimetic total synthesis of indole alkaloids using alkyne-based strategies
Significance of nonbiomimetic natural product synthesis and nonbiomimetic total syntheses of indole alkaloids based on the construction of core structures using alkyne reactions are summarized in this review.
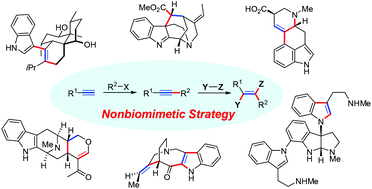
Org. Biomol. Chem., 2021,19, 3551-3568
https://doi.org/10.1039/D0OB02577A
Stereoselective syntheses and biological activities of E-series resolvins
Total syntheses of E-series resolvins are reviewed, along with the most significant bioactions of the E-series resolvins.
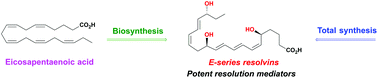
Org. Biomol. Chem., 2021,19, 705-721
https://doi.org/10.1039/D0OB02218G
Recent advances in the synthesis of plakortin-type polyketides
The recent progress in the synthesis of plakortin-type polyketides is summarized, with an emphasis on the key elements enabling rapid assembly of the polycyclic cores of chased targets.
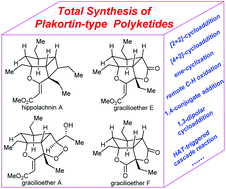
Org. Biomol. Chem., 2020,18, 9371-9384
https://doi.org/10.1039/D0OB01930E
Emergence of 2,3,5-trisubstituted tetrahydrofuran natural products and their synthesis
Diverse syntheses of emerging 2,3,5-trisubstituted-THF natural products are reviewed encompassing strategies based on catalytic methods, cascade reactions and rearrangements.
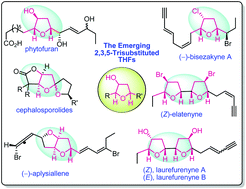
Org. Biomol. Chem., 2020,18, 7002-7025
https://doi.org/10.1039/D0OB01542C
Total synthesis of natural products using photocycloaddition reactions of arenes
The photocycloaddition reaction of benzene with alkenes has become a significant approach for organic chemists and thus has been frequently utilized as a key step in the total synthesis of natural products.
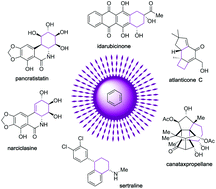
Org. Biomol. Chem., 2020,18, 5558-5566
https://doi.org/10.1039/D0OB01204A
Strategies for the construction of γ-spirocyclic butenolides in natural product synthesis
This review describes the stereoselective synthesis of a γ-spirocyclic containing natural products or model compounds showing phytotoxic, antibiotic, anti-inflammatory, antiviral, antimicrobial, cytotoxic, and antitumor activities.

Org. Biomol. Chem., 2020,18, 5287-5314
https://doi.org/10.1039/D0OB00954G
Palladium-catalyzed asymmetric dearomative cyclization in natural product synthesis
This review discusses palladium-catalyzed asymmetric dearomative cyclization, which is invaluable for producing enantioenriched materials, for complex natural product synthesis.
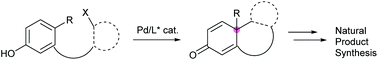
Org. Biomol. Chem., 2020,18, 4354-4370
https://doi.org/10.1039/D0OB00818D
Synthetic approaches towards cortistatins: evolution and progress through its ages
With an exhaustive coverage of all the synthetic approaches towards cortistatins, this review serves to assemble most of the avenues which have been employed to conquer this complex steroidal alkaloid architecture.
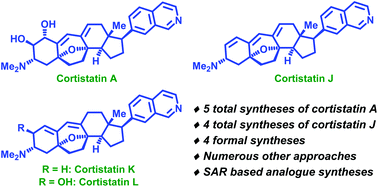
Org. Biomol. Chem., 2020,18, 3965-3995
https://doi.org/10.1039/D0OB00770F
Asymmetric copper-catalyzed conjugate additions of organometallic reagents in the syntheses of natural compounds and pharmaceuticals
Stereoselective copper-catalyzed conjugate additions of organometallic reagents serve as a versatile tool in the total syntheses of diverse natural compounds and pharmaceutical agents.
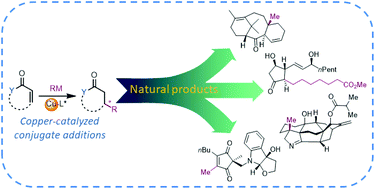
Org. Biomol. Chem., 2020,18, 3780-3796
https://doi.org/10.1039/D0OB00278J
Synthetic pathways to tetrahydrocannabinol (THC): an overview
This review summarises various synthetic pathways leading to tetrahydrocannabinol and structurally related cannabinoids.

Org. Biomol. Chem., 2020,18, 3203-3215
https://doi.org/10.1039/D0OB00464B
Recent progress in the total synthesis of Strychnos alkaloids
The recent synthetic approaches toward Strychnos alkaloids are summarized, including novel synthetic methodologies and strategies.
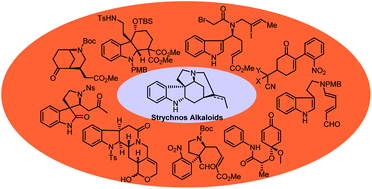
Org. Biomol. Chem., 2020,18, 1046-1056
https://doi.org/10.1039/C9OB02627D
Recent progress in the total synthesis of marine brominated sesquiterpene aplydactone
In this review, we summarize five instructive total syntheses of marine brominated sesquiterpene aplydactone developed over the past five years.
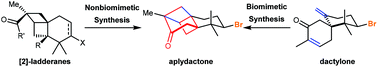
Org. Biomol. Chem., 2020,18, 1036-1045
https://doi.org/10.1039/C9OB02642H
Synthetic approaches towards avibactam and other diazabicyclooctane β-lactamase inhibitors
The synthetic strategies to obtain avibactam and other diazabicyclooctane β-lactamase inhibitors such as ETX2514 are presented.
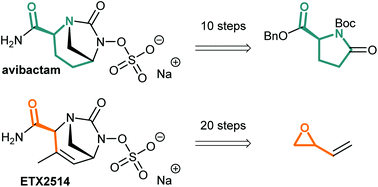
Org. Biomol. Chem., 2020,18, 830-844
https://doi.org/10.1039/C9OB02605C
Full solid-phase total synthesis of macrocyclic natural peptides using four-dimensionally orthogonal protective groups
This review focuses on four-dimensionally orthogonal protective group strategies for the full solid-phase synthesis of macrocyclic natural peptides.
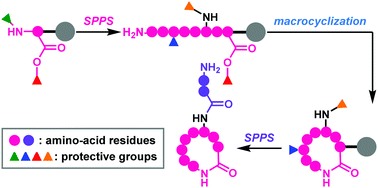
Org. Biomol. Chem., 2019,17, 6519-6527
https://doi.org/10.1039/C9OB01130G
Versatility of glycals in synthetic organic chemistry: coupling reactions, diversity oriented synthesis and natural product synthesis
Versatility of glycals in the stereoselective synthesis of natural products.
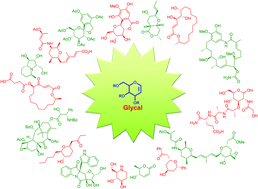
Org. Biomol. Chem., 2019,17, 4153-4182
https://doi.org/10.1039/C9OB00343F
Progress in total synthesis of subincanadine alkaloids and their congeners
Total synthesis of subincanadine alkaloids and their biogenetic congeners are described in a focused manner.
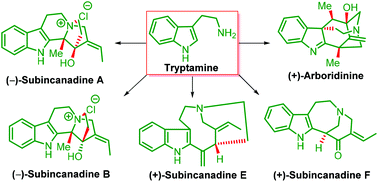
Org. Biomol. Chem., 2019,17, 745-761
https://doi.org/10.1039/C8OB02565G
Recent advances in the synthesis of azaphenalene alkaloids: first enantioselective approaches
This review provides an update of the synthetic progress towards azaphenalene alkaloids, including the pioneering enantioselective approaches.
Org. Biomol. Chem., 2018,16, 8218-8229
https://doi.org/10.1039/C8OB01443D
Natural products as modulators of the cyclic-AMP pathway: evaluation and synthesis of lead compounds
Natural product modulators of the cAMP pathway have been evaluated and their total synthesis campaign is described in detail.
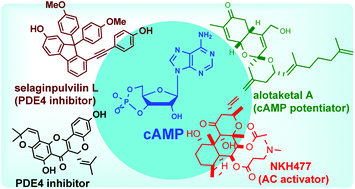
Org. Biomol. Chem., 2018,16, 6372-6390
https://doi.org/10.1039/C8OB01388H
Review of synthetic approaches toward maoecrystal V
Synthetic approaches toward the complex natural product diterpenoid maoecrystal V are reviewed, including successful total syntheses, published synthetic efforts, and efforts compiled from dissertations.
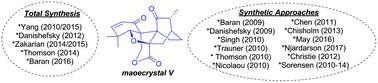
Org. Biomol. Chem., 2018,16, 4210-4222
https://doi.org/10.1039/C8OB00909K
Total synthesis of complex alkaloids by nucleophilic addition to amides
This mini review focuses on the recent progress of total synthesis of complex alkaloids based on the nucleophilic additions to N-alkoxyamides, tertiary amides and secondary amides.
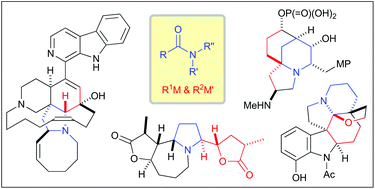
Org. Biomol. Chem., 2018,16, 3864-3875
https://doi.org/10.1039/C8OB00733K
Construction of azaspirocyclic skeletons mediated by the carbonyl of the Weinreb amide: formal total synthesis of (±)-cephalotaxine
A facile Stevens rearrangement of the Weinreb amide and the subsequent key steps mediated by the carbonyl of the Weinreb amide led to the construction of azaspirocyclic skeletons of some typical alkaloids.
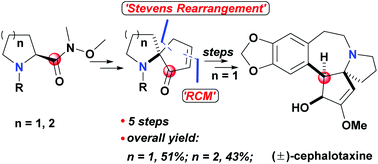
Org. Biomol. Chem., 2022,20, 1879-1882
https://doi.org/10.1039/D1OB02304G
Total syntheses of (+)-adunctins C and D: assignment of their absolute configurations
The first asymmetric synthesis of adunctins C and D was achieved, thus enabling the assignment of the absolute configurations of these two natural products.
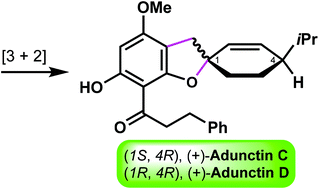
Org. Biomol. Chem., 2021,19, 9840-9843
https://doi.org/10.1039/D1OB02055B
Toward the synthesis of the hypoxia selective anticancer agent BE-43547 A2
The stereoselective synthesis of the 19-epi-BE-43547 A2 chiral framework is achieved from D-glucose by employing an unusual (Z)-selective Julia–Kocienski olefination and hydroboration as key steps.
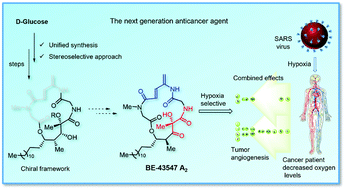
Org. Biomol. Chem., 2021,19, 9833-9839
https://doi.org/10.1039/D1OB01824H
Synthesis of tetracyclic spiroindolines by an interrupted Bischler–Napieralski reaction: total synthesis of akuammicine
The interrupted Bischler–Napieralski reaction of β,γ-unsaturated tryptamides affords tetracyclic spiro pyrroloindolines, which can be used in the total synthesis of the Strychnos alkaloid, akuammicine.
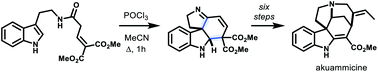
Org. Biomol. Chem., 2021,19, 9641-9644
https://doi.org/10.1039/D1OB01966J
A multifunctional divergent scaffold to access the formal syntheses of various sesquiterpenoids
A new divergent scaffold for the synthesis of various sesquiterpenoids in furan and α,β-unsaturated lactone oxidation states was developed through a novel, concise and scalable route.
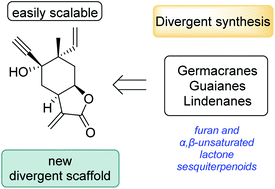
Org. Biomol. Chem., 2021,19, 8687-8690
https://doi.org/10.1039/D1OB01716K
A three-step enantioselective synthesis of (+)- and (−)-α-thujone
An asymmetric synthesis of α-thujone was achieved with a gold-catalyzed cycloisomerization as the key step. The route provides access either enantiomer of the natural product without the use of protecting groups or redox manipulations.
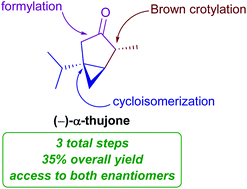
Org. Biomol. Chem., 2021,19, 8018-8020
https://doi.org/10.1039/D1OB01505B
Ambruticins: tetrahydropyran ring formation and total synthesis
The total synthesis of ambruticin J and epoxidation/cyclisation studies on model unsaturated hydroxy esters are described.
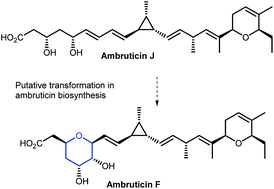
Org. Biomol. Chem., 2021,19, 6210-6215
https://doi.org/10.1039/D1OB00883H
Total syntheses of melodienones by redox isomerization of propargylic alcohols
A remarkable base-promoted methodology for the rapid construction of the (E)- and (Z)-γ-oxo-α,β-alkenoic ester skeletons from readily accessible vinyl propargylic alcohols through modified redox isomerization was uncovered.
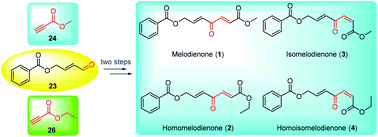
Org. Biomol. Chem., 2021,19, 5077-5081
https://doi.org/10.1039/D1OB00599E
Synthesis of an isomer of lycoplanine A via cascade cyclization to construct the spiro-N,O-acetal moiety
A 6/10/5/5 tetracyclic isomer of lycoplanine A is synthesized using D–A reaction and cascade cyclization to respectively establish the [9.2.2] pentadecane skeleton and spirocenter, providing sufficient experience to synthesize lycoplanine A.
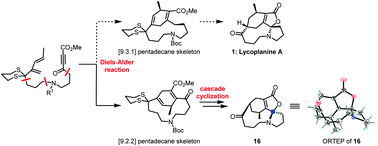
Org. Biomol. Chem., 2021,19, 1748-1751
https://doi.org/10.1039/D0OB02399J
Enantioselective total synthesis of (+)-ieodomycin A, (+)-ieodomycin B, and their three stereoisomers
Enantioselective routes for the total synthesis of ieodomycins A & B, and three stereochemical analogues of ieodomycin B via a late-stage elaboration of the side chain.
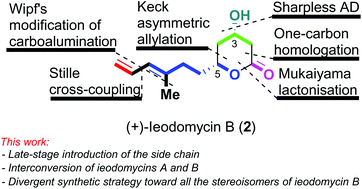
Org. Biomol. Chem., 2020,18, 9227-9230
https://doi.org/10.1039/D0OB02107E
Total synthesis of thioamycolamide A via a biomimetic route
A concise total synthesis of thioamycolamide A was accomplished as a key step of thio-Michael addition via a biomimetic route.
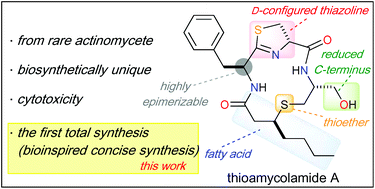
Org. Biomol. Chem., 2020,18, 8366-8370
https://doi.org/10.1039/D0OB01942A
Total synthesis of tubulysin U and N14-desacetoxytubulysin H
A concise and efficient procedure for the total synthesis of tubulysin U and N14-desacetoxytubulysin H has been developed with high stereoselectivity on a gram scale.
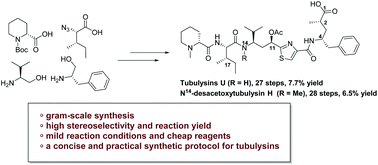
Org. Biomol. Chem., 2020,18, 5349-5353
https://doi.org/10.1039/D0OB01109F
Stereoselective synthesis of an eleganine A core
The core structure of eleganine A – a cytotoxic indole monoterpene alkaloid – was accessed for the first time.
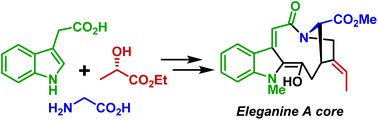
Org. Biomol. Chem., 2020,18, 4566-4568
https://doi.org/10.1039/D0OB00939C
Synthesis of B-ring-fluorinated (−)-epicatechin gallate derivatives
Electronically modified, fluorinated catechins and epicatechins are enantioselectively synthesized in a short, convergent sequence via kinetic resolution.
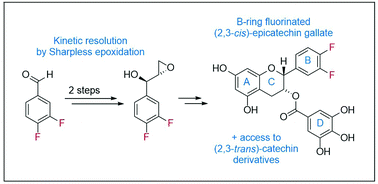
Org. Biomol. Chem., 2020,18, 4024-4028
https://doi.org/10.1039/D0OB00686F
Divergent syntheses of okaramines C, J, L, and S–U
The first total synthesis of okaramines L and S–U, and the concise synthesis of okaramines C and J have been accomplished using a common intermediate bromohexahydropyrroloindole in 5–8 steps with an overall yield of 6.7%–23.0%.
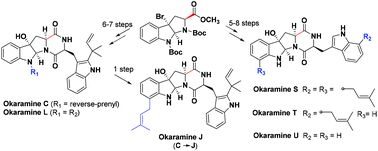
Org. Biomol. Chem., 2020,18, 3848-3852
https://doi.org/10.1039/D0OB00587H
Total synthesis of tricolorin A via interrupted Pummerer reaction-mediated glycosylation and one-pot relay glycosylation
The total synthesis of tricolorin A was achieved with high efficiency based on interrupted Pummerer reaction-mediated (IPRm) glycosylation and one-pot relay glycosylation.
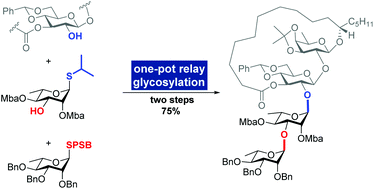
Org. Biomol. Chem., 2020,18, 3818-3822
https://doi.org/10.1039/D0OB00513D
Total synthesis of isatindigotindoline C
Total synthesis of isatindigotindoline C is achieved in two steps using a biomimetic 1,3-dipolar cycloaddition reaction as the key step.
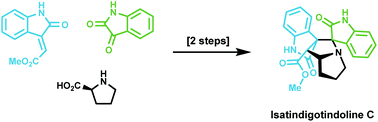
Org. Biomol. Chem., 2020,18, 2051-2053
https://doi.org/10.1039/D0OB00270D
Syntheses of doubly linked proanthocyanidins using free flavan units as nucleophiles: insight into the origin of the high regioselectivity of annulation
Reported herein is a viable synthesis route to procyanidins A1 and A2, doubly linked flavan dimers. The use of nascent nucleophilic partner, catechin or epicatechin, enabled facile regioselective annulation with an ethylenedioxy-bridged flavan unit.
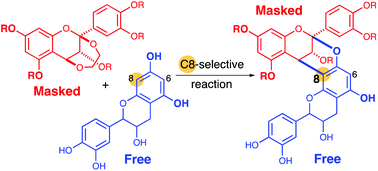
Org. Biomol. Chem., 2019,17, 9129-9134
https://doi.org/10.1039/C9OB01896D
Facile access to evodiakine enabled by aerobic copper-catalyzed oxidative rearrangement
Oxidative rearrangement for the first rapid construction of evodiakine was developed.
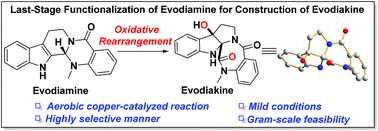
Org. Biomol. Chem., 2019,17, 8811-8815
https://doi.org/10.1039/C9OB01832H
Total syntheses of seminolipid and its analogues by using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent
Total synthesis of seminolipid was accomplished via regioselective protection using 2,6-bis(trifluoromethyl)phenylboronic acid followed by regioselective trichloroethyl-protected sulfation as key steps.
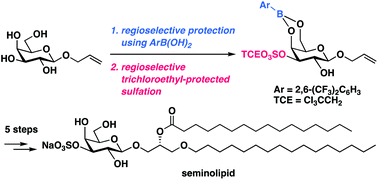
Org. Biomol. Chem., 2019,17, 7325-7329
https://doi.org/10.1039/C9OB01445D
Total synthesis of (+)-ar-macrocarpene
First total synthesis of a naturally occurring sesquiterpenoid, (+)-ar-macrocarpene, has been achieved via a key [3,3]-sigmatropic rearrangement effecting reductive transposition through allylic diazene rearrangement (ADR).
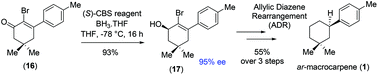
Org. Biomol. Chem., 2019,17, 7140-7143
https://doi.org/10.1039/C9OB01373C
A biomimetic approach towards phorone sesterterpenoids
We report efforts towards a unified total synthesis of Korean sponge derived sesterterpenoids phorones A and B, using a biomimetic strategy. This work has established a new synthetic approach to the parent ansellane sesterterpenoid skeleton with unanticipated diversion along a biogenetically related pathway.
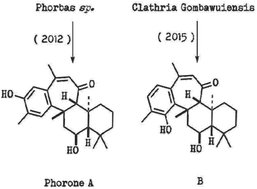
Org. Biomol. Chem., 2019,17, 6985-6988
https://doi.org/10.1039/C9OB00745H
Total synthesis of (±)-epi-stemodan-13α,17-diol
Stemodan-13α,17-diol is a natural stemodane-type diterpenoid isolated from Stemodia chilensis.
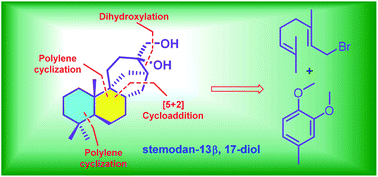
Org. Biomol. Chem., 2019,17, 4711-4714
https://doi.org/10.1039/C9OB00661C
A new route to tricyclane sesquiterpenoids: total synthesis of α-ekasantalic acid
Chemical manipulation of the cycloadduct of citraconic anhydride and cyclopentadiene enables a new synthetic route to tricyclane sesquiterpenoids.
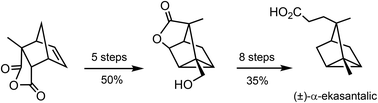
Org. Biomol. Chem., 2019,17, 4456-4459
https://doi.org/10.1039/C9OB00630C
Total synthesis of (±)-antroquinonol
We report the total synthesis of (±)-antroquinonol based on a concise and efficient route.
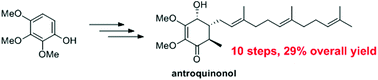
Org. Biomol. Chem., 2019,17, 1754-1757
https://doi.org/10.1039/C8OB02494D
Palladium-catalyzed intramolecular carboborylation of 1,3-diene and synthesis of ABCD ring of communesins
A palladium-catalyzed intramolecular carboborylation of 1,3-diene has been developed for the synthesis of iminoindolines with a quaternary carbon centre.
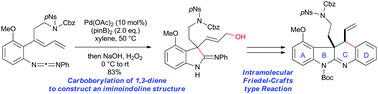
Org. Biomol. Chem., 2019,17, 1731-1735
https://doi.org/10.1039/C8OB02224K
Synthesis of the core structure of phalarine
Two new palladium-catalyzed reactions enabled the synthesis of the core structure of phalarine.
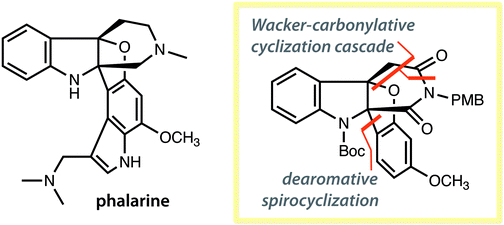
Org. Biomol. Chem., 2019,17, 1727-1730
https://doi.org/10.1039/C8OB02320D
Total synthesis of incargranine A
Synthetic studies into the origins of the alkaloid incargranine A have resulted in the development of a four-step (longest linear sequence) total synthesis.
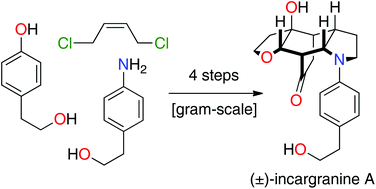
Org. Biomol. Chem., 2019,17, 1698-1702
https://doi.org/10.1039/C8OB00702K
Synthesis and biological evaluation of fluorinated analogues of ripostatin A
A last stage click-chemistry approach to synthesize the heterocycle-containing derivatives of fluorinated ripostatin A was developed.
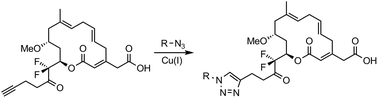
Org. Biomol. Chem., 2019,17, 1362-1364
https://doi.org/10.1039/C8OB02890G
Enantioselective synthesis and absolute configuration determination of hydroxywilfordic acid in sesquiterpene pyridine alkaloids
An enantioselective synthetic route to hydroxywilfordic acid, a key subunit of sesquiterpene pyridine alkaloids such as wilfortrine, was developed.
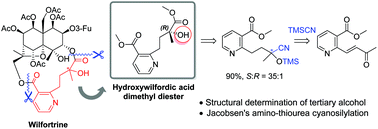
Org. Biomol. Chem., 2019,17, 44-48
https://doi.org/10.1039/C8OB02364F
Total synthesis of the potent anti-inflammatory natural product solomonamide A along with structural revision and biological activity evaluation
Herein, we report the total synthesis of solomonamide A along with its structural revision for the first time.
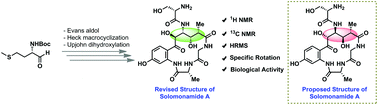
Org. Biomol. Chem., 2018,16, 9138-9142
https://doi.org/10.1039/C8OB02713G
Syntheses of the plant auxin conjugate 2-O-(indole-3-acetyl)-myo-inositol IAInos
The two first chemical syntheses of IAInos (2-O-(indole-3-acetyl)-myo-inositol), an important plant hormone, are described.
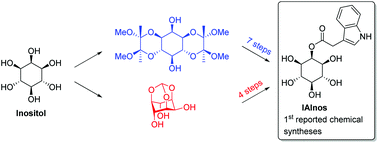
Org. Biomol. Chem., 2018,16, 6860-6864
https://doi.org/10.1039/C8OB02096E
Semi-syntheses of the 11-hydroxyrotenoids sumatrol and villosinol
We describe semi-syntheses of the 11-hydroxyrotenoids sumatrol (1) and villosinol (2), starting from rotenone (5), using an oxime-directed C11–H functionalisation approach.

Org. Biomol. Chem., 2018,16, 6395-6398
https://doi.org/10.1039/C8OB01919C
Biomimetic total syntheses of chromane meroterpenoids, guadials B and C, guapsidial A and psiguajadial D
The first biomimetic total syntheses of chromane meroterpenoids, guadials B and C, guapsidial A and psiguajadial D have been completed.
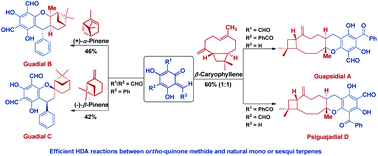
Org. Biomol. Chem., 2018,16, 4793-4796
https://doi.org/10.1039/C8OB01092G
Synthesis of two new gem-fluoronitro containing tetranitroadamantanes and property comparison with their nitro and gem-dinitro analogues
Two new fluorinated tetranitroadamantanes were synthesized and their physical properties were compared with their analogues on the basis of experimental and calculated results.
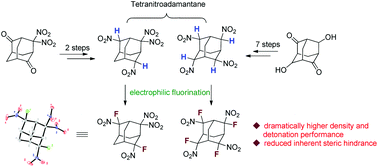
Org. Biomol. Chem., 2018,16, 4784-4788
https://doi.org/10.1039/C8OB01140K
Synthesis of the [7-5-5] tricyclic core of Daphniphyllum alkaloids
The [7-5-5] tricyclic core of the Daphniphyllum alkaloids was constructed, featuring a Claisen-Ireland rearrangement to install the two contiguous stereogenic centers, E1cB elimination to form the tetrasubstituted C–C double bond, and a 2,3-Wittig rearrangement to construct the quaternary carbon.
![Graphical abstract: Synthesis of the [7-5-5] tricyclic core of Daphniphyllum alkaloids](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB00859K&imageInfo.ImageIdentifier.Year=2018)
Org. Biomol. Chem., 2018,16, 3556-3559
https://doi.org/10.1039/C8OB00859K
Stereoselective preparation of key intermediates for the synthesis of iso-, neuro- and phyto-prostane family members: inaugural asymmetric synthesis of 17-E2c-dihomo- and 17-F2c-dihomo-isoprostanes
A practical synthesis of enantiopure key intermediates for the preparation of prostanoids has been described.
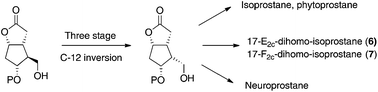
Org. Biomol. Chem., 2018,16, 2393-2396
https://doi.org/10.1039/C8OB00489G
Diversity-oriented routes to thiopeptide antibiotics: total synthesis and biological evaluation of micrococcin P2
The first synthesis of micrococcin P2 has been achieved by late-stage Suzuki coupling of a macrocyclic boronic acid with a 2-bromothiazole.
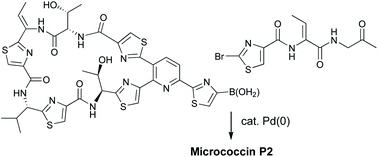
Org. Biomol. Chem., 2022,20, 1893-1899
https://doi.org/10.1039/D1OB02145A
Progress toward a biomimetic synthesis of pegaharmaline A
Efforts to validate the proposed biosynthesis of pegaharmaline A led to the formation of several new heteroaromatic scaffolds.
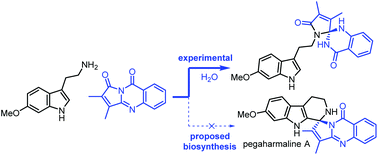
Org. Biomol. Chem., 2022,20, 1275-1283
https://doi.org/10.1039/D1OB02372A
A unified total synthesis of benzo[d][1,3]dioxole-type benzylisoquinoline alkaloids of aporphines, coptisines, and dibenzopyrrocolines
A unified approach leading to benzo[d][1,3]dioxole-type benzylisoquinoline alkaloids of aporphines, coptisines, and dibenzopyrrocolines.
![Graphical abstract: A unified total synthesis of benzo[d][1,3]dioxole-type benzylisoquinoline alkaloids of aporphines, coptisines, and dibenzopyrrocolines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB02258J&imageInfo.ImageIdentifier.Year=2022)
Org. Biomol. Chem., 2022,20, 658-666
https://doi.org/10.1039/D1OB02258J
Studies directed toward the synthesis of hedycoropyrans: total synthesis of des-hydroxy (−)-hedycoropyran B (ent-rhoiptelol B)
Studies directed towards the stereoselective synthesis of the diarylheptanoid-derived natural products hedycoropyrans leading to the total synthesis of (−)-hedycoropyran B (ent-rhoiptelol B) are presented.
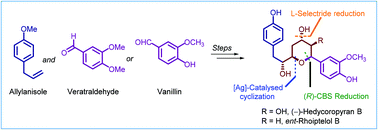
Org. Biomol. Chem., 2022,20, 444-463
https://doi.org/10.1039/D1OB01972D
Synthesis of illudalic acid and analogous phosphatase inhibitors
A convergent 5-step synthesis (LLS) of illudalic acid allows for concise preparation of analogues for pharmacological evaluation.
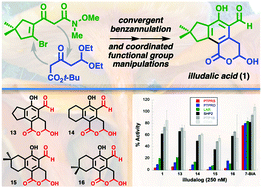
Org. Biomol. Chem., 2021,19, 10596-10600
https://doi.org/10.1039/D1OB02106K
Diastereoselective synthesis of (±)-trichodiene and (±)-trichodiene-D3 as analytical standards for the on-site quantification of trichothecenes
A new RCM-based, divergent synthetic strategy towards native and deuterium labeled (±)-trichodiene is presented.
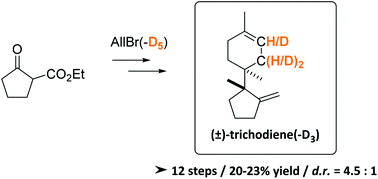
Org. Biomol. Chem., 2021,19, 9872-9879
https://doi.org/10.1039/D1OB01778K
A modular strategy for the synthesis of marine originated meroterpenoid-type natural products
Chemoselective formal synthesis of (−)-pelorol and 9-epi-pelorol was achieved by controlling the reaction sequence of hydrogenation and cyclization. Synthesis of (+)-yahazunone and (+)-yahazunol was also accomplished using palladium-catalyzed tandem carbene migratory insertion.
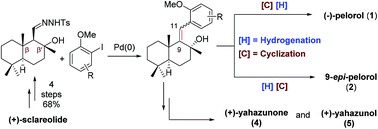
Org. Biomol. Chem., 2021,19, 9439-9447
https://doi.org/10.1039/D1OB01598B
Stereocomplementary synthesis of casuarine and its 6-epi-, 7-epi-, and 6,7-diepi-stereoisomers
A stereocomplementary strategy allows the efficient synthesis of casuarine, 6-epi-casuarine, 7-epi-casuarine, and 6,7-diepi-casuarine from a cyclic nitrone and a nitrone-derived aldehyde. Their glycosidase inhibition profiles were compared.
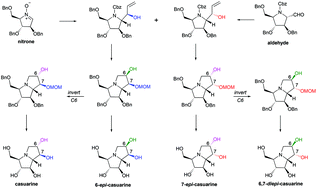
Org. Biomol. Chem., 2021,19, 9410-9420
https://doi.org/10.1039/D1OB01725J
About this collection
The latest articles published in Organic and Biomolecular Chemistry related to total synthesis.