A modular strategy for the synthesis of marine originated meroterpenoid-type natural products†
Abstract
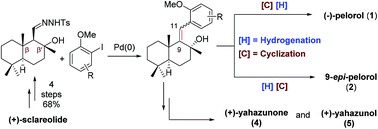
A modular strategy for meroterpenoid-type marine natural products has been developed from commercially available (+)-sclareolide using a palladium-catalyzed tandem carbene migratory insertion as one of the key steps. Its applicability is showcased by the formal synthesis of (−)-pelorol and 9-epi-pelorol and the concise total synthesis of (+)-yahazunone and (+)-yahazunol. It is worth noting that the formal synthesis of (−)-pelorol and 9-epi-pelorol was achieved by controlling the reaction sequence of hydrogenation and cyclization.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...