Total synthesis of complex alkaloids by nucleophilic addition to amides
Abstract
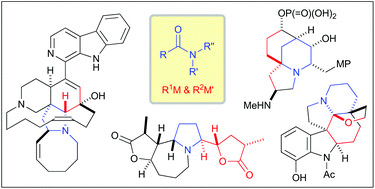
Nucleophilic addition to amides has been recognized as a promising transformation for total synthesis of complex alkaloids. Amides can accept two different organometallic reagents through the nucleophilic addition, which enables it to serve as a stable surrogate of multi-substituted amines. However, the nucleophilic addition has been overlooked for a long time due to three main reasons: low electrophilicity of amide carbonyls, potential hydrolysis of the reaction intermediate and excess addition of an organometallic reagent. This mini review focuses on the recent progress of total synthesis of complex alkaloids based on the nucleophilic additions to N-alkoxyamides, tertiary amides and secondary amides.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...