Toward the synthesis of the hypoxia selective anticancer agent BE-43547 A2†
Abstract
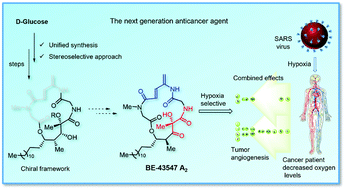
A short and enantioselective synthesis of the 19-epi-BE-43547 A2 chiral framework has been achieved in a high yield. The challenging key C15 tertiary stereocenter was derived from D-glucose. The synthetic strategy involves a Julia–Kocienski olefination to install the lipophilic side chain. An efficient protocol for Z to E isomerization of olefin was developed using a novel UV flow reactor. In addition, an unprecedented oxygen mediated hydroboration and the Krapcho decarboxylation of β-keto lactone were observed.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...