Synthesis of illudalic acid and analogous phosphatase inhibitors†
Abstract
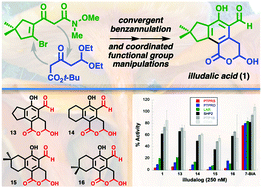
Developing an efficient, concise synthesis of the fungal natural product illudalic acid has been a long-standing challenge, made more pressing by the recent discovery that illudalic acid and analogs are selective phosphatase inhibitors. Syntheses of illudalic acid have become progressively more efficient over the decades yet remain strategically grounded in a 17-step synthesis reported in 1977. Here we validate a two-step process—convergent [4 + 2] benzannulation and one-pot coordinated functional group manipulations—for preparing the key trifunctional pharmacophore of illudalic acid. The modular building blocks are readily available in 2–3 steps, for a longest linear sequence (LLS) of 5 steps to illudalic acid from 3,3-dimethylcyclopentanone. A small collection of analogous indanes and tetralins featuring the same pharmacophore were prepared by a similar route. These compounds potently and selectively inhibit the human leukocyte common antigen-related (LAR) subfamily of protein tyrosine phosphatases (PTPs). Evidence supporting a postulated covalent ligation mechanism is provided herein.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...