Total synthesis of (±)-epi-stemodan-13α,17-diol†
Abstract
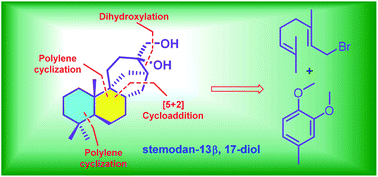
Stemodan-13α,17-diol is a natural stemodane-type diterpenoid isolated from Stemodia chilensis. Herein we report the total synthesis of its epimer, stemodan-13β,17-diol, by applying titanium-mediated polyene cyclization and iron-catalyzed [5 + 2] cycloaddition as the key transformations to expeditiously install the molecular scaffold.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...