Palladium-catalyzed asymmetric dearomative cyclization in natural product synthesis
Abstract
Asymmetric catalysis is a rapidly growing field in modern organic chemistry and has been indispensable for the synthesis of enantioenriched materials to meet demands from the academies to pharmaceutical industries. Asymmetric dearomative cyclization catalyzed by transition metals has been a hot research area in the last decade. Fascinated by its ability to construct sterically hindered quaternary stereogenic center(s) through dearomatization and simultaneously forging new ring structure(s) through cyclization, palladium-catalyzed asymmetric dearomative cyclization has been applied to the synthesis of structurally complicated natural products and it is increasingly prevalent in the literature. In particular, the resultant product from dearomative cyclization, which usually carries one or more unsaturated C–C bond(s), allows further functional group transformations. Previously reported applications of palladium-catalyzed asymmetric dearomative cyclization in natural product synthesis are presented here and discussed in depth.
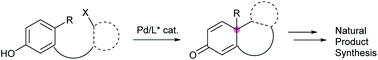
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...