Themed collection Contemporary Synthetic Chemistry in Drug Discovery

Fluorination methods in drug discovery
Late stage fluorination methods applied to biologically-active drugs have provided the pharmaceutical industry with new leads that show improved properties such as modulation of lipophilicity, electronegativity, basicity, bioavailability, and deceleration of metabolic degradation.
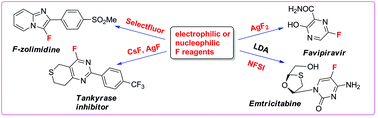
Org. Biomol. Chem., 2016,14, 8398-8427
https://doi.org/10.1039/C6OB00764C
Late stage trifluoromethylthiolation strategies for organic compounds
The introduction of CF3S groups into compounds with known biological activity can alter their properties significantly, as a result of the increased lipophilicity and electronegativity of the trifluoromethylthio group.
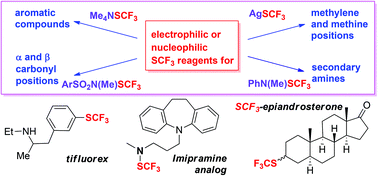
Org. Biomol. Chem., 2016,14, 7150-7182
https://doi.org/10.1039/C6OB00763E
The xanthate route to organofluorine derivatives. A brief account
The radical chemistry of xanthates allows numerous approaches to organofluorine compounds.
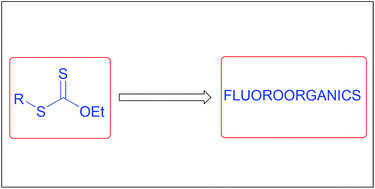
Org. Biomol. Chem., 2016,14, 6891-6912
https://doi.org/10.1039/C6OB01087C
Modern advances in heterocyclic chemistry in drug discovery
New advances in functionalized heterocyclic chemistry are of critical importance to the medicinal chemist as it provides the ability to expand the available drug-like chemical space and drive more efficient delivery of drug discovery programs.
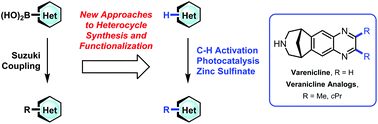
Org. Biomol. Chem., 2016,14, 6611-6637
https://doi.org/10.1039/C6OB00936K
Enantioselective synthesis of cyanohydrins catalysed by hydroxynitrile lyases – a review
Ever since their first application in 1908, HNLs have been gaining strength in enantioselective cyanohydrin synthesis.
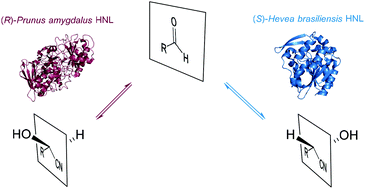
Org. Biomol. Chem., 2016,14, 6375-6389
https://doi.org/10.1039/C6OB00934D
Total synthesis, biosynthesis and biological profiles of clavine alkaloids
This review highlights noteworthy synthetic and biological aspects of the clavine subfamily of ergot alkaloids.
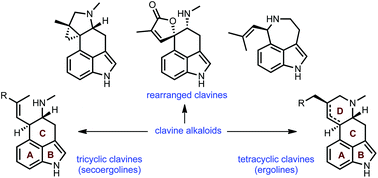
Org. Biomol. Chem., 2016,14, 5894-5913
https://doi.org/10.1039/C6OB00878J
A practical metal-free homolytic aromatic alkylation protocol for the synthesis of 3-(pyrazin-2-yl)bicyclo[1.1.1]pentane-1-carboxylic acid
As a part of our ongoing synthetic quest to expand the frontiers of contemporary medicinal chemistry, we now report an expedient synthesis of a potentially useful bicyclo[1.1.1]pentane building block, 3-(pyrazin-2-yl)bicyclo[1.1.1]pentane-1-carboxylic acid. This report also showcases the application of this motif as a “spacer” probe in a biological study.
![Graphical abstract: A practical metal-free homolytic aromatic alkylation protocol for the synthesis of 3-(pyrazin-2-yl)bicyclo[1.1.1]pentane-1-carboxylic acid](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB01799A&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 9485-9489
https://doi.org/10.1039/C6OB01799A
Copper-catalysed oxidative amination of quinoxalin-2(1H)-ones with aliphatic amines
A novel, efficient and practical method for copper-catalysed oxidative C-3 amination of quinoxalin-2(1H)-ones with primary or secondary amines as the nitrogen sources has been developed.
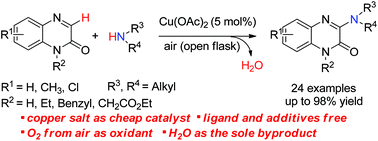
Org. Biomol. Chem., 2016,14, 8428-8432
https://doi.org/10.1039/C6OB01283C
Synthesis of “neoprofen”, a rigidified analogue of ibuprofen, exemplifying synthetic methodology for altering the 3-D topology of pharmaceutical substances
The unique hydrophobic 3-D topology of the neopentylene ring fusion is explored in the context of an analogue of the non-steroidal anti-inflammatory drug, ibuprofen.
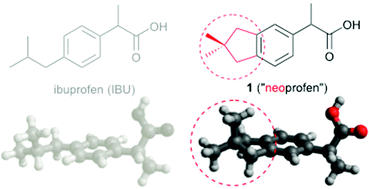
Org. Biomol. Chem., 2016,14, 7855-7858
https://doi.org/10.1039/C6OB01351A
A metal-catalyzed enyne-cyclization step for the synthesis of bi- and tricyclic scaffolds amenable to molecular library production
A facile metal-catalyzed diversification step for the synthesis of novel bi- and tricyclic scaffolds from enyne substrates is reported in this study for molecular library production.
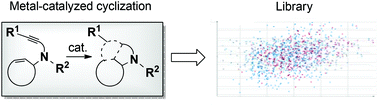
Org. Biomol. Chem., 2016,14, 6947-6950
https://doi.org/10.1039/C6OB01148A
Enantioselective N-heterocyclic carbene-catalyzed synthesis of saccharine-derived dihydropyridinones with cis-selectivity
The N-heterocyclic carbene-catalyzed [2 + 4] cyclocondensation of α-chloroaldehydes and saccharine-derived 1-azadienes was developed, giving dihydropyridinones in good yields with exclusive cis-selectivities and excellent enantioselectivities.
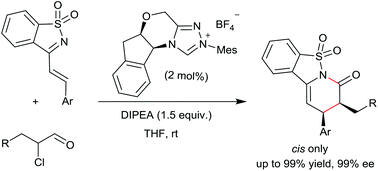
Org. Biomol. Chem., 2016,14, 6422-6425
https://doi.org/10.1039/C6OB01040G
Photooxygenation of an amino-thienopyridone yields a more potent PTP4A3 inhibitor
Late-stage photooxygenation can generate novel biologically active lead structures.

Org. Biomol. Chem., 2016,14, 6398-6402
https://doi.org/10.1039/C6OB00946H
Scalable procedure for the fragmentation of hydroperoxides mediated by copper and iron tetrafluoroborate salts
An improved protocol for the formal elimination of propene from organic substrates is reported.
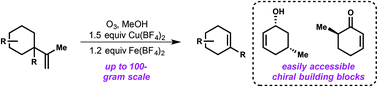
Org. Biomol. Chem., 2016,14, 6197-6200
https://doi.org/10.1039/C6OB00941G
Synthesis of annulated pyridines as inhibitors of aldosterone synthase (CYP11B2)
A series of potent and selective aldosterone synthase (CYP11B2) inhibitors were prepared in one step through an intermolecular Kondrat'eva reaction.
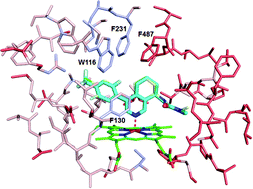
Org. Biomol. Chem., 2016,14, 5922-5927
https://doi.org/10.1039/C6OB00848H
Rh-catalysed [5 + 1] cycloaddition of allenylcyclopropanes and CO: reaction development and application to the formal synthesis of (−)-galanthamine
A Rh-catalyzed [5 + 1] cycloaddition of allenylcyclopropanes and CO has been developed to synthesize functionalized 2-methylidene-3,4-cyclohexenones. This cycloaddition reaction has been utilized as a key step in the formal synthesis of natural product (−)-galanthamine.
![Graphical abstract: Rh-catalysed [5 + 1] cycloaddition of allenylcyclopropanes and CO: reaction development and application to the formal synthesis of (−)-galanthamine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00660D&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5945-5950
https://doi.org/10.1039/C6OB00660D
An asymmetric approach to bicyclo[2.2.1]heptane-1-carboxylates via a formal [4 + 2] cycloaddition reaction enabled by organocatalysis
An organocatalytic formal [4 + 2] cycloaddition reaction has been developed that permits rapid access to bicyclo[2.2.1]heptane-1-carboxylates with excellent enantioselectivities under mild and operationally simple conditions.
![Graphical abstract: An asymmetric approach to bicyclo[2.2.1]heptane-1-carboxylates via a formal [4 + 2] cycloaddition reaction enabled by organocatalysis](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00814C&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 5229-5232
https://doi.org/10.1039/C6OB00814C
Base-catalyzed controllable reaction of 3-ylideneoxindoles with O-Boc hydroxycarbamates for the synthesis of amidoacrylates and spiroaziridine oxindoles
A base-catalyzed divergent reaction of 3-ylideneoxindoles and O-Boc hydroxycarbamates has been developed to provide various amidoacrylates and spiroaziridine oxindoles with high yields.
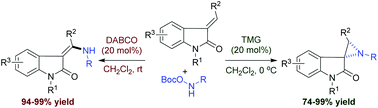
Org. Biomol. Chem., 2016,14, 5224-5228
https://doi.org/10.1039/C6OB00891G
The total synthesis and functional evaluation of fourteen stereoisomers of yaku'amide B. The importance of stereochemistry for hydrophobicity and cytotoxicity
Yaku'amide B is a highly unsaturated linear tridecapeptide and an extremely potent cytotoxin.
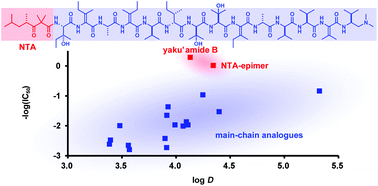
Org. Biomol. Chem., 2016,14, 4199-4204
https://doi.org/10.1039/C6OB00640J
Synthesis of cycloalkyl substituted purine nucleosides via a metal-free radical route
The selective synthesis of C6-monocycloalkyl or C6,C8-dicycloalkyl substituted purine nucleosides could be realized. Furthermore, uracil and related nucleosides were also suitable substrates, giving the C5-cyclohexyl substituted uracil derivatives in good yields with excellent regioselectivities.
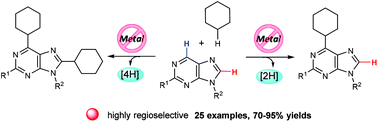
Org. Biomol. Chem., 2016,14, 4189-4193
https://doi.org/10.1039/C6OB00596A
Identification of an active metabolite of PAR-1 antagonist RWJ-58259 and synthesis of analogues to enhance its metabolic stability
Understanding the pharmacokinetic behaviour of PAR-1 antagonist RWJ-58259 and the synthesis of analogues to enhance metabolic stability.

Org. Biomol. Chem., 2016,14, 3198-3201
https://doi.org/10.1039/C6OB00332J
Oxidative coupling between C(sp2)–H and C(sp3)–H bonds of indoles and cyclic ethers/cycloalkanes
Cross-dehydrogenative-coupling (CDC) between C–H/C–H bonds of indoles and cyclic ethers/cycloalkanes is made viable through a simple transition-metal-free pathway.
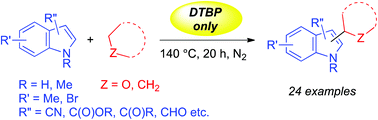
Org. Biomol. Chem., 2016,14, 2608-2612
https://doi.org/10.1039/C6OB00076B
Radical fluorination powered expedient synthesis of 3-fluorobicyclo[1.1.1]pentan-1-amine
This work describes an expedient synthesis of a potentially useful BCP derivative, 3-fluorobicyclo[1.1.1]pentan-1-amine, by employing a contemporary radical fluorination method.
![Graphical abstract: Radical fluorination powered expedient synthesis of 3-fluorobicyclo[1.1.1]pentan-1-amine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5OB02066B&imageInfo.ImageIdentifier.Year=2015)
Org. Biomol. Chem., 2015,13, 11597-11601
https://doi.org/10.1039/C5OB02066B
Tetrazolone as an acid bioisostere: application to marketed drugs containing a carboxylic acid
Matched molecular pair analysis was used to evaluate the ability of a tetrazolone group to act as a bioisostere of a carboxylic acid.
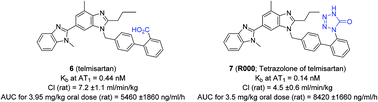
Org. Biomol. Chem., 2016,14, 9343-9347
https://doi.org/10.1039/C6OB01646D
A one-pot synthesis of tetrazolones from acid chlorides: understanding functional group compatibility, and application to the late-stage functionalization of marketed drugs
A one-pot and scalable synthesis of tetrazolones (tetrazol-5-ones) from acid chlorides using azidotrimethylsilane is presented.

Org. Biomol. Chem., 2016,14, 9338-9342
https://doi.org/10.1039/C6OB01644H
Synthesis of trifluoromethylthiolated pyridinones through the copper-mediated trifluoromethylthiolation of iodopyridinones
A simple method for the synthesis of trifluoromethylthiolated pyridinones through the copper-mediated trifluoromethylthiolation of iodopyridinones was developed.
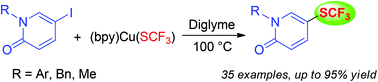
Org. Biomol. Chem., 2016,14, 8615-8622
https://doi.org/10.1039/C6OB01348A
Ravynic acid, an antibiotic polyeneyne tetramic acid from Penicillium sp. elucidated through synthesis
Ravynic acid, an unsaturated tetramic acid possessing antibiotic activity, has been isolated from the fungus Penicillium sp. and identified through synthesis.
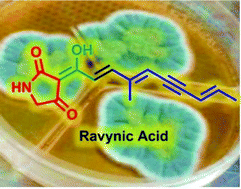
Org. Biomol. Chem., 2016,14, 8253-8260
https://doi.org/10.1039/C6OB00938G
Palladium-catalyzed β-(hetero)arylation of α,β-unsaturated valerolactams
A method for the palladium-catalyzed arylation of α,β-unsaturated valerolactams is reported.
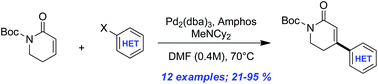
Org. Biomol. Chem., 2016,14, 7731-7734
https://doi.org/10.1039/C6OB01126H
Horner–Wadsworth–Emmons approach to piperlongumine analogues with potent anti-cancer activity
Horner–Wadsworth–Emmons approach to a selection of piperlongumine-like compounds from a novel phosphonoacetamide reagent.
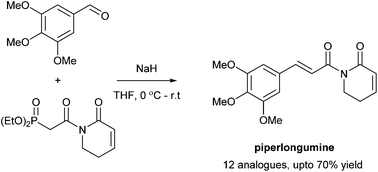
Org. Biomol. Chem., 2016,14, 7585-7593
https://doi.org/10.1039/C6OB01160H
Synthesis of cyclopenta-fused polycyclic aromatic hydrocarbons utilizing aryl-substituted anilines
Cyclopenta-fused polycyclic aromatic hydrocarbons were synthesized from readily available 2-aryl-substituted anilines under extremely mild conditions.
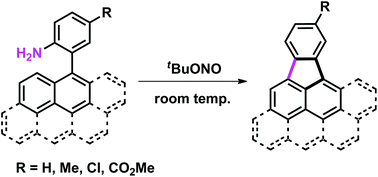
Org. Biomol. Chem., 2016,14, 6804-6810
https://doi.org/10.1039/C6OB01235C
Regioselective routes to orthogonally-substituted aromatic MIDA boronates
A series of tetrasubstituted aromatics has been synthesized, many of which are based on elaborated N-methyliminodiacetic acid (MIDA)-boronates.
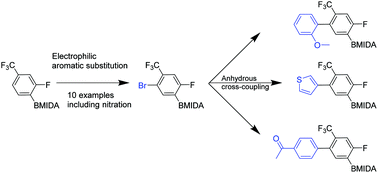
Org. Biomol. Chem., 2016,14, 6751-6756
https://doi.org/10.1039/C6OB01141A
Synthesis of trifluoromethylated isoxazoles and their elaboration through inter- and intra-molecular C–H arylation
We report conditions for the preparation of a range of trifluoromethylated isoxazole building blocks through the cycloaddition reaction of trifluoromethyl nitrile oxide and their subsequent derivatisation.
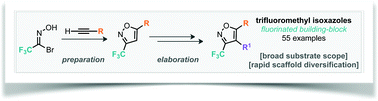
Org. Biomol. Chem., 2016,14, 5983-5991
https://doi.org/10.1039/C6OB00970K
Synthesis of furo[3,2-c]coumarin derivatives using visible-light-promoted radical alkyne insertion with bromocoumarins
Heterocyclic framework furo[3,2-c]coumarins were constructed using a visible-light promoted photoredox neutral coupling of 3-bromo-4-hydroxycoumarins with commercially available alkynes.
![Graphical abstract: Synthesis of furo[3,2-c]coumarin derivatives using visible-light-promoted radical alkyne insertion with bromocoumarins](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6OB00768F&imageInfo.ImageIdentifier.Year=2016)
Org. Biomol. Chem., 2016,14, 6065-6070
https://doi.org/10.1039/C6OB00768F
Iron-mediated oxidative C–H coupling of arenes and alkenes directed by sulfur: an expedient route to dihydrobenzofurans
A route to medicinally-relevant dihydrobenzofurans utilises a sulfur-directed, iron-mediated C–H ortho-coupling of arenes and alkenes, and a palladium-catalysed heterocyclisation.
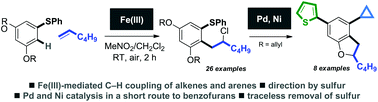
Org. Biomol. Chem., 2016,14, 5286-5292
https://doi.org/10.1039/C6OB00883F
UV light-mediated difunctionalization of alkenes with CF3SO2Na: synthesis of trifluoromethyl phenanthrene and anthrone derivatives
A metal-free and cost-effective protocol for UV light-mediated difunctionalization of alkenes with CF3SO2Na was developed.
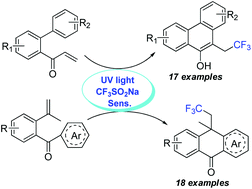
Org. Biomol. Chem., 2016,14, 5293-5297
https://doi.org/10.1039/C6OB00912C
Palladium-catalyzed direct desulfitative C2 arylations of 3-halo-N-protected indoles using (hetero)arenesulfonyl chlorides
Halo-substituents at the indolyl C3 position act as temporary blocking groups allowing the regioselective formation of 2-arylindoles through a direct desulfitative arylation. This method allows one to prepare a wide variety of indole derivatives in a few steps.
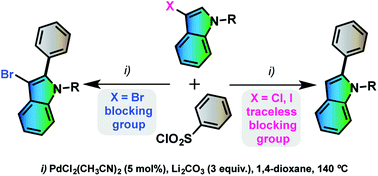
Org. Biomol. Chem., 2016,14, 4947-4956
https://doi.org/10.1039/C6OB00584E
Optimization and multigram scalability of a catalytic enantioselective borylative migration for the synthesis of functionalized chiral piperidines
A systematic optimization of a Pd-catalyzed enantioselective borylative migration of an alkenyl nonaflate derivative of the simple precursor, N-Boc-4-piperidone, was achieved.
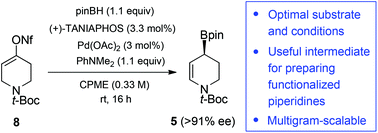
Org. Biomol. Chem., 2016,14, 4739-4748
https://doi.org/10.1039/C6OB00685J
Metal free C–H functionalization of diazines and related heteroarenes with organoboron species and its application in the synthesis of a CDK inhibitor, meriolin 1
Here, we report a metal-free cross-coupling reaction of diazines and related heteroarenes with organoboron species.
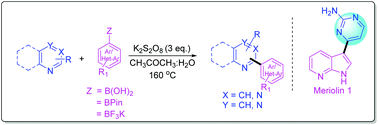
Org. Biomol. Chem., 2016,14, 4312-4320
https://doi.org/10.1039/C6OB00526H
Synthesis of cyclic 1,3-diols as scaffolds for spatially directed libraries
A series of 1,3-diols has been synthesized and used to create a pilot library of spatially diverse compounds.
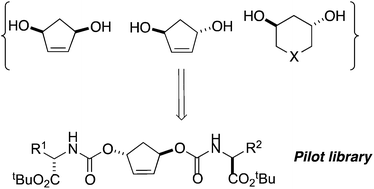
Org. Biomol. Chem., 2016,14, 4299-4303
https://doi.org/10.1039/C6OB00598E
Enantioselective organocatalytic Michael addition of isorhodanines to α,β-unsaturated aldehydes
The Michael reaction of substituted isorhodanines with α,β-unsaturated aldehydes in the presence of a catalytic amount of a chiral secondary amine is presented.
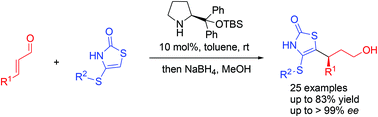
Org. Biomol. Chem., 2016,14, 3926-3933
https://doi.org/10.1039/C5OB02457A
Synthesis and absolute configuration assignment of albucidin: a late-stage reductive deiodination by visible light photocatalysis
The total synthesis and absolute configuration assignment of albucidin might pave the way for synthetic and medicinal chemists for further research on this type of bioactive molecule.

Org. Biomol. Chem., 2016,14, 3482-3485
https://doi.org/10.1039/C6OB00371K
Conformation-induced regioselective and divergent opening of epoxides by fluoride: facile access to hydroxylated fluoro-piperidines
Utilizing molecular conformation as a controlling factor, epoxide-containing 2-aryl-piperidines can be ring-opened with the reagent combination of TBAF/KHF2 in a regioselective and divergent fashion.

Org. Biomol. Chem., 2016,14, 3469-3475
https://doi.org/10.1039/C6OB00063K
Iron-mediated inter- and intramolecular reductive cross-coupling of unactivated alkyl chlorides with aryl bromides
Efficient inter- and intramolecular reductive cross-coupling of unactivated alkyl chlorides by Fe(PPh3)2Cl3.

Org. Biomol. Chem., 2016,14, 3314-3321
https://doi.org/10.1039/C6OB00247A
An atom-efficient and convergent approach to the preparation of NS5A inhibitors by C–H activation
A novel approach of the convergent functionalisation of aryl dibromides to form NS5A type inhibitors using C–H activation is reported.
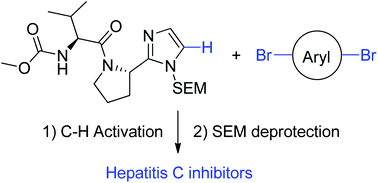
Org. Biomol. Chem., 2016,14, 3307-3313
https://doi.org/10.1039/C6OB00340K
Synthesis of novel and potent vorapaxar analogues
Unlocking novel and potent vorapaxar analogues by functionalisation of previously unexplored positions on the parent vorapaxar scaffold.
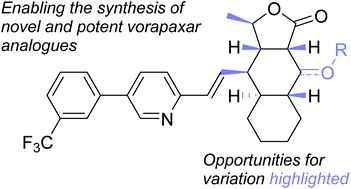
Org. Biomol. Chem., 2016,14, 3264-3274
https://doi.org/10.1039/C5OB02541A
Catalytic selective deuteration of halo(hetero)arenes
A library of deuterated compounds was synthesized and the mechanism of D-incorporation explored.
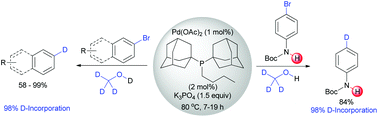
Org. Biomol. Chem., 2016,14, 3091-3097
https://doi.org/10.1039/C6OB00193A
Enzymatic transhalogenation of dendritic RGD peptide constructs with the fluorinase
The fluorinase enzyme is used to catalyse transhalogenation reactions on dendritic RGD peptide constructs. The strategy is explored for [18F]-radiolabelling of peptides under neutral aqueous ambient conditions for positron emission tomography (PET).
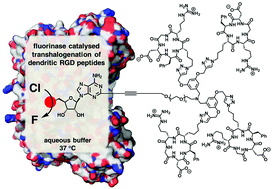
Org. Biomol. Chem., 2016,14, 3120-3129
https://doi.org/10.1039/C6OB00239K
Flexible synthesis of polyfunctionalised 3-fluoropyrroles
Convergent synthesis of polyfunctionalised 3-fluoropyrroles.
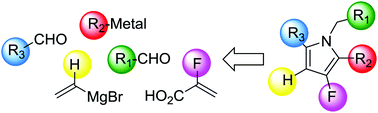
Org. Biomol. Chem., 2016,14, 183-190
https://doi.org/10.1039/C5OB02155C
About this collection
This high quality collection aims to demonstrate the importance of synthetic chemistry in drug discovery. Specifically it will highlight how synthetic methods can be used to address hurdles currently being faced in modern medicinal chemistry.
It has been guest edited by Prof. Angela Russell (University of Oxford, UK), Prof. Douglas E. Frantz (The University of Texas at San Antonio, USA) Dr Graham Wynne (University of Oxford, UK) Dr Matthew Duncton (Rigel, San Francisco, USA) and Dr Shane Krska (Merck, Boston, USA).
New articles will be added to this collection as they are published.