Synthesis of trifluoromethylthiolated pyridinones through the copper-mediated trifluoromethylthiolation of iodopyridinones†
Abstract
An operationally simple method for the copper-mediated trifluoromethylthiolation of iodopyridinones employing (bpy)CuSCF3 (1; bpy = 2,2′-bipyridine) as a trifluoromethylthiolating reagent is presented. Various types of iodopyridinones are applicable and the trifluoromethylthiolated pyridinones are obtained in moderate to excellent yields. This method tolerates a variety of protecting groups on the nitrogen atom of pyridinones. In addition, scalability of the reaction is demonstrated.
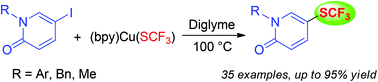
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...