Optimization and multigram scalability of a catalytic enantioselective borylative migration for the synthesis of functionalized chiral piperidines†
Abstract
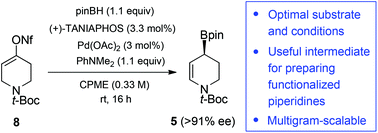
The development of new, efficient and economical methods for the preparation of functionalized, optically enriched piperidines is important in the field of drug discovery where this class of heterocycles is often deemed a privileged structure. We have optimized a Pd-catalyzed enantioselective borylative migration of an alkenyl nonaflate derivative of the simple precursor, N-Boc-4-piperidone. This anomalous borylation reaction lends access to a chiral optically enriched piperidinyl allylic boronate that can be employed in carbonyl allylboration and stereoselective cross-coupling to produce substituted dehydropiperidines related to numerous pharmaceutical agents. A systematic fine-tuning of reaction conditions revealed that diethyl ether and the green solvent cyclopentyl methyl ether are suitable reaction solvents providing the highest enantioselectivity (up to 92% ee) under a low catalyst loading of 3 mol%. Optimization of the aldehyde allylboration step led to higher yields with further solvent economy. The multigram-scalability of the entire process was demonstrated under the reaction conditions that provide optimal atom-economy and efficiency.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...