Synthesis of annulated pyridines as inhibitors of aldosterone synthase (CYP11B2)†
Abstract
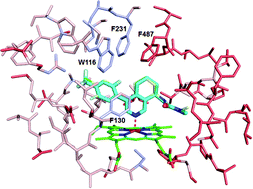
A series of cyclopenta[c]pyridine aldosterone synthase (AS) inhibitors were conveniently accessed using batch or continuous flow Kondrat'eva reactions. Preparation of the analogous cyclohexa[c]pyridines led to the identification of a potent and more selective AS inhibitor. The structure-activity-relationship (SAR) in this new series was rationalized using binding mode models in the crystal structure of AS.
- This article is part of the themed collection: Contemporary Synthetic Chemistry in Drug Discovery


 Please wait while we load your content...
Please wait while we load your content...