Flexible synthesis of polyfunctionalised 3-fluoropyrroles†
Received
19th October 2015
, Accepted 5th November 2015
First published on 5th November 2015
Abstract
An efficient and selective approach for the synthesis of polyfunctionalised 3-fluoropyrroles has been developed starting from commercial aldehydes. The methodology is concise, efficient and allows for the modular and systematic assembly of polysubstituted 3-fluoropyrroles. This synthesis provides an alternative and highly convergent strategy for the generation of these chemically and biologically important units.
Introduction
Polyfunctionalised pyrroles are an integral part of medicinal chemistry, forming the core unit of a number of biologically active compounds.1–4 Fluorinated polyfunctionalised pyrroles are particularly interesting due to their useful biological, metabolic, physical and pharmacokinetic properties. Key fluorinated pyrroles include compounds such as 1 and 2 which have been developed as anti-inflammatory and anti-hypertension agents respectively (Fig. 1).5,6
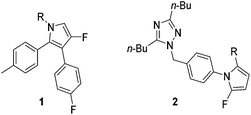 |
| | Fig. 1 Biologically active fluorinated pyrrole examples. Pyrrole 1 has anti-inflammatory activity, while pyrrole 2 is used to alleviate hypertension.1,2 | |
Thus, it is not surprising that a significant amount of interest has been devoted to the synthesis of fluorinated pyrroles in recent years.7–9 As such, new flexible and efficient methods for their syntheses are desired.
Fluorinated α,β-unsaturated lactams were first synthesised via a ring-closing metathesis approach by Haufe and co-workers.10 The work was extended by the groups of Rutjes and Marquez to produce a number of novel fluorinated compounds (Scheme 1). To date, most work has been centred on the synthesis of 5 and 6-membered lactams with various degrees of functionalisation.11,12 We feel fluorinated α,β-unsaturated γ-lactams would be the ideal building block to provide access to polyfunctionalised fluorinated pyrroles.
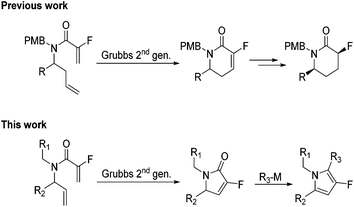 |
| | Scheme 1 Previous work to generate fluorinated δ-lactams via RCM approach. | |
Herein, we would like to report a quick, flexible and modular synthesis of polyfunctionalised fluorinated pyrroles. The methodology allows for the systematic introduction of substituents to produce novel polyfunctionalised fluorinated building blocks (Scheme 1).
Results and discussion
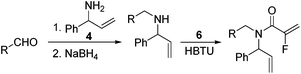
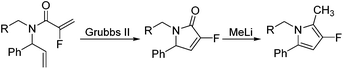
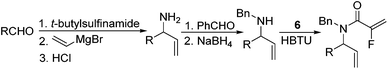
Our initial approach to the synthesis of the pyrrole core began with the condensation of benzaldehyde 3 with t-butylsulfinamide to generate the corresponding imine, which upon vinylation with vinylmagnesium bromide afforded the desired allylic amine 4 in excellent yield.13 Reductive amination of amine 4 with p-anisaldehyde then produced the PMB protected amine 5 in high yield (Scheme 2).
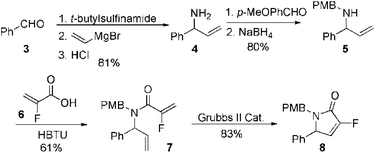 |
| | Scheme 2 Synthesis of fluorinated α,β-unsaturated lactam 8. | |
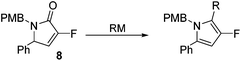
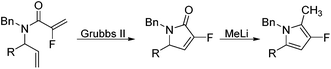
Coupling of amine 5 with 2-fluoroacrylic acid 6 then proceeded to produce the desired amide unit 7 in reasonable yield. Subsequent treatment of diene 7 with Grubbs 2nd gen. catalyst then afforded the expected α,β-unsaturated lactam 8 in good yield.12
Rewardingly, alkylation of fluorolactam 8 with methyllithium proceeded cleanly to generate the desired pyrrole unit 9 in excellent yield (Scheme 3).14,15 Mechanistically, we believe that this aromatisation process takes place through hemi-aminal formation followed by elimination of water and double bond isomerisation.15,16
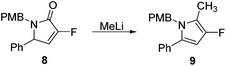 |
| | Scheme 3 Synthesis of fluorinated polysubstituted pyrrole 9. | |
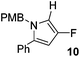
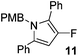
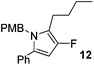
The alkylation–aromatisation methodology was then expanded by including an array of nucleophiles as to allow for the selective introduction of substituents at the C2 position of the C3 fluorinated-pyrrole ring. Thus, a collection of organometallic reagents including DIBAL-H, n-butyllithium, phenyllithium and allylmagnesium bromide were used to generate the desired substituted pyrroles 10–13 in high yields (Table 1).14–16
Table 1 Introduction of different nucleophiles (RM) in the synthesis of tetrasubstituted pyrroles (9–13)
Having demonstrated the ability to incorporate substituents at the pyrrole C2 position through an alkylation–aromatisation process, it was decided to explore the ability of our methodology to incorporate substituents in the other pyrrole positions.
Thus, it was decided to showcase the methodology by generating a number of C3 fluorinated pyrrole analogues with different N-substituents. Synthetically, the generation of the new analogues was envisioned as originating through the incorporation of different aldehyde units during the reductive amination step.
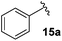
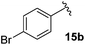
Following this approach, different aromatic substitution patterns were initially explored with the benzyl and 4-bromobenzyl derivatives 14a and 14b being cleanly converted to the RCM precursors 15a and 15b in high yields (Table 2).
Table 2 Synthesis of fluorinated diene-amides 15a–d
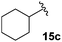
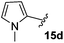
The non-aromatic derivative 15c, bearing a cyclohexylmethyl group, worked well with yields upwards of 80% for both steps. N-Methylpyrrole-2-carboxaldehyde was also cleanly incorporated, yielding the desired diene 15d in good yield over the sequence.
Ring-closing metathesis was then successfully carried out in all cases, with isolated yields higher than 80%.17 Gratifyingly, treatment of pyrrolidone compounds 16a–d with methyllithium under our alkylation–aromatisation methodology afforded the desired N-substituted pyrrole derivatives 17a–d in excellent isolated yields (Table 3).
Table 3 Synthesis of fluorinated pyrroles 17a–d
At this point, it was decided to now focus on exploring the nature and effect of the starting aldehyde on our pyrrole forming sequence. By changing the identity of the starting aldehyde, a range of functional groups could be efficiently installed at the C5 position (Table 4).
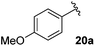
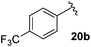
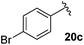
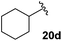
Table 4 Synthesis of fluorinated amides 20a–e
|
|
| R |
Yield |
|
Yield |
|
Yield |
|
| 4-MeOC6H4 |
71% |
18a
|
79% |
19a
|
60% |
20a
|
| 4-CF3C6H4 |
61% |
18b
|
80% |
19b
|
45% |
20b
|
| 4-BrC6H4 |
75% |
18c
|
84% |
19c
|
62% |
20c
|
| Cyclohexyl |
50% |
18d
|
70% |
19d
|
76% |
20d
|
| PhCH2CH2 |
58% |
18e
|
88% |
19e
|
75% |
20e
|
Electron donating and withdrawing aromatic analogues were investigated, resulting in good yields of the allylic amine intermediates 18a–c. Aliphatic aldehydes could also be converted to the corresponding primary amines 18d–e in reasonable yields.18 Treatment of the crude allylic amines 18a–e under reductive amination conditions afforded the secondary amines 19a–e which upon coupling with 2-fluoroacrylic acid 6 generated the desired fluorinated amides 20a–20e in good yields.
Ring-closing metathesis in all cases proceeded in high yields (Table 5). However, examples with electron withdrawing substituents required extended reaction times and higher catalyst loadings (15 mol%) to achieve high yields. Gratifyingly, treatment of all the pyrrolidone intermediates (21a–21e) under the methyllithium promoted alkylation–aromatisation conditions yielded the desired fluorinated tetrasubstituted pyrroles 22a–e in good to excellent yield.
Table 5 Synthesis of fluorinated pyrroles 22a–d
Conclusion
In conclusion, we have developed an efficient and selective approach for the synthesis of polyfunctionalised 3-fluorinated pyrroles. The methodology is concise and allows for the modular synthesis of chemically and biologically important units.
Experimental
All reactions were performed in oven-dried glassware under an inert argon atmosphere unless otherwise stated. Tetrahydrofuran (THF), diethyl ether, toluene and dichloromethane (DCM) were purified through a solvent purification system. Petroleum ether refers to the fraction boiling between 40–60 °C. All reagents were used as received, unless otherwise stated. Solvents were evaporated under reduced pressure at 40 °C unless otherwise stated. IR spectra were recorded as thin films on NaCl plates using a Fourier Transform spectrometer. Only significant absorptions (νmax) are reported in wavenumbers (cm−1). Proton magnetic resonance spectra (1H NMR) were recorded at either 400 or 500 MHz. Fluorine magnetic resonance spectra (19F NMR) were recorded at either 377 or 470 MHz. Carbon magnetic resonance spectra (13C NMR) were recorded at either 100 or 125 MHz. Chemical shifts (δ) are reported in parts per million (ppm) and are referenced to the residual solvent peak. The order of citation in parentheses is (1) number of equivalent nuclei (by integration), (2) multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, sext = sextet, sept = septet, m = multiplet, b = broad), (3) and coupling constant (J) quoted in Hertz to the nearest 0.1 Hz. High resolution mass spectra were obtained by electrospray (EI) chemical ionisation (CI) mass spectrometery operating at a resolution of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 full widths at half height. Flash chromatography was performed using silica gel (40–63 micron) as the stationary phase. TLC was performed on aluminium sheets pre-coated with silica (Silica Gel 60 F254) unless otherwise stated. The plates were visualised by the quenching of UV fluorescence (λmax 254 nm) and/or by staining with either anisaldehyde, potassium permanganate, iodine or cerium ammonium molybdate followed by heating.
000 full widths at half height. Flash chromatography was performed using silica gel (40–63 micron) as the stationary phase. TLC was performed on aluminium sheets pre-coated with silica (Silica Gel 60 F254) unless otherwise stated. The plates were visualised by the quenching of UV fluorescence (λmax 254 nm) and/or by staining with either anisaldehyde, potassium permanganate, iodine or cerium ammonium molybdate followed by heating.
General procedure I
A solution of the diene (1 eq.) in toluene (0.005 g ml−1) and was heated to 100 °C. Grubbs 2nd generation catalyst was added in portions and the reaction was stirred until completion as indicated by TLC analysis. The reaction was cooled down to room temperature, the solvent was removed under reduced pressure and the crude material was purified by flash column chromatography.
General procedure II
α,β-Unsaturated lactam (1 eq.) was dissolved in diethyl ether (5 mL) and cooled to 0 °C. Methyllithium (1.1 eq.) was added dropwise and the mixture was stirred for 1 h. Following this time, the reaction was quenched with H2O (10 mL), extracted with diethyl ether (3 × 10 mL), dried (Na2SO4) and evaporated in vacuo. The crude residue was purified by flash column chromatography.
3-Fluoro-1-[(4′-methoxyphenyl)methyl]-5-phenyl-2,5-dihydro-1H-pyrrol-2-one, 8.
Dialkene 7 (270 mg, 0.84 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–15% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 8 (210 mg, 0.71 mmol, 83%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.50–7.33 (3H, m), 7.20–7.09 (2H, m), 7.06 (2H, JH = 8.6 Hz), 6.85 (2H, d, JH = 8.6 Hz), 6.26 (1H, d, JH = 1.6 Hz), 5.12 (1H, d, JH = 14.8 Hz), 4.78 (1H, dd, JF = 6.0 Hz, JH = 2.4 Hz), 3.82 (3H, m), 3.58 (1H, JH = 14.8 Hz). 19F NMR (CDCl3, 470 MHz) δ: −138.5. 13C NMR (CDCl3, 125 MHz) δ: 163.0 (d, JF = 31.2 Hz), 159.2, 152.3 (d, JF = 279.6 Hz), 133.9 (d, JF = 2.1 Hz), 129.8 (2C), 129.3 (2C), 129.2, 128.6, 127.6 (2C), 118.4 (d, JF = 4.4 Hz), 114.2 (2C), 59.1 (d, JF = 5.7 Hz), 55.3, 43.5. m/z [EI (+ve)] 297.2 [M]+, HRMS found [M]+ 297.1164, C18H16FNO2 requires 297.1165. IR (thin film) vmax = 2355, 1710, 1666, 1514, 1247 cm−1.
3-Fluoro-1-[(4′-methoxyphenyl)methyl]-2-methyl-5-phenyl-1H-pyrrole, 9.
α,β-Unsaturated lactam 8 (36 mg, 0.12 mmol) was reacted with methyl lithium (83 μL, 0.13 mmol, 1.6 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 9 (30 mg, 0.1 mmol, 86%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.35–7.13 (5H, m), 6.78–6.76 (4H, m), 5.96 (1H, s), 4.93 (2H, s), 3.72 (3H, s), 1.98 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −169.4. 13C NMR (CDCl3, 125 MHz) δ: 158.7, 149.2 (d, JF = 235.6 Hz), 132.9, 130.6, 130.1 (d, JF = 6.9 Hz), 128.8 (2C), 128.5 (2C), 127.1, 126.7 (2C), 114.2 (2C), 112.5 (d, JF = 24.3 Hz), 96.4 (d, JF = 16.4 Hz), 55.3, 47.2, 8.2. m/z [EI (+ve)] 295.2 [M]+, HRMS found [M]+ 295.1373 C19H18FNO requires 295.1372. IR (thin film) vmax = 2928, 2359, 1614, 1599, 1512, 1352, 1249, 1174 cm−1. m.p. 73–75 °C.
4-Fluoro-1-[(4′-methoxyphenyl)methyl]-2-phenyl-1H-pyrrole, 10.
α,β-Unsaturated lactam 8 (40 mg, 0.13 mmol) was dissolved in CH2Cl2 (4 mL) and cooled to −78 °C. Diisobutylaluminium hydride (0.41 mL, 0.41 mmol, 1 M in hexanes) was added dropwise and the mixture was stirred for 16 h. Following this time, the reaction was quenched with H2O (10 mL), extracted with diethyl ether (3 × 10 mL), dried (Na2SO4) and evaporated in vacuo. The crude residue was purified be flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 10 (30 mg, 0.11 mmol, 85%) as a yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.40–7.32 (5H, m), 6.98 (2H, d, JH = 8.7 Hz), 6.86 (2H, d, JH = 8.7 Hz), 6.46 (1H, dd, JF = 3.2 Hz, JH = 2.0 Hz), 6.04 (1H, d, JF = 2.4 Hz), 4.99 (2H, s), 3.82 (3H, s). 19F NMR (CDCl3, 470 MHz) δ: −165.4. 13C NMR (CDCl3, 125 MHz) δ: 159.0, 152.0 (d, JF = 239.1 Hz), 132.5 (d, JF = 1.6 Hz), 131.8 (d, JF = 6.4 Hz), 130.2, 129.0 (2C), 128.5 (2C), 127.9 (2C), 127.5, 114.1 (2C) 105.5 (d, JF = 27.3 Hz), 97.1 (d, JF = 16.4 Hz), 55.3, 50.2. m/z [EI (+ve)] 281.1 [M]+, HRMS found [M]+ 281.1215, C18H16FNO requires 281.1216. IR (thin film) vmax = 2956, 2837, 1701, 1612, 1512, 1247, 1176 cm−1.
3-Fluoro-1-[(4′-methoxyphenyl)methyl]-2,5-diphenyl-1H-pyrrole, 11.
α,β-Unsaturated lactam 8 (45 mg, 0.15 mmol) was dissolved in diethyl ether (5 mL) and cooled to 0 °C. Phenyllithium (87 μL, 0.16 mmol, 1.9 M in di-n-butyl ether) was added dropwise and the mixture was stirred for 1 h. Following this time, the reaction was quenched with H2O (10 mL), extracted with diethyl ether (3 × 10 mL), dried (Na2SO4) and evaporated in vacuo. The crude residue was purified be flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 11 (50 mg, 0.14 mmol, 93%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.39–7.36 (7H, m), 7.35–7.37 (3H, m), 6.66 (2H, d, JH = 8.8 Hz), 6.55 (2H, d, JH = 8.8 Hz), 6.17 (1H, br s), 5.10 (2H, s), 3.74 (3H, s). 19F NMR (CDCl3, 470 MHz) δ: −165.3. 13C NMR (CDCl3, 125 MHz) δ: 158.5, 149.6 (d, JF = 242.5 Hz), 133.0 (d, JF = 7.1 Hz), 132.9 (d, JF = 1.8 Hz), 130.8, 129.9 (d, JF = 3.3 Hz), 129.5, 129.1 (2C), 128.5 (2C), 128.5 (2C), 127.5, 127.3 (2C), 127.2, 119.1 (d, JF = 21.0 Hz), 113.7 (2C), 98.4 (d, JF = 16.6 Hz), 55.2, 48.3. m/z [EI (+ve)] 357.0 [M]+, HRMS found [M]+ 357.1531, C24H20FNO requires 357.1529. IR (thin film) vmax = 3063, 2955, 2835, 1610, 1512, 1492, 1435, 1247, 1176 cm−1. m.p. 84–86 °C.
2-Butyl-3-fluoro-1-[(4′-methoxyphenyl)methyl]-5-phenyl-1H-pyrrole, 12.
α,β-Unsaturated lactam 8 (38 mg, 0.13 mmol) was dissolved in diethyl ether (5 mL) and cooled to 0 °C. n-Butyllithium (54 μL, 0.13 mmol, 2.5 M in hexanes) was added dropwise and the mixture was stirred for 1 h. Following this time, the reaction was quenched with H2O (10 mL), extracted with diethyl ether (3 × 10 mL), dried (Na2SO4) and evaporated in vacuo. The crude residue was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 12 (34 mg, 0.10 mmol, 78%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.22–7.14 (5H, m), 6.74 (4H, m), 5.95 (1H, s), 4.94 (2H, s), 3.71 (3H, s), 2.37 (2H, t, JH = 7.6 Hz), 1.43–1.34 (2H, m), 1.25–1.18 (2H, m), 0.78 (3H, t, JH = 7.3 Hz). 19F NMR (CDCl3, 470 MHz) δ: −168.0. 13C NMR (CDCl3, 125 MHz) δ: 158.7, 149.5 (d, JF = 236.1 Hz), 133.0, 131.0, 130.0 (d, JF = 7.0 Hz), 128.9 (2C), 128.4 (2C), 127.1, 126.7 (2C), 117.0 (d, JF = 23.5 Hz), 114.1 (2C), 96.7 (d, JF = 16.6 Hz), 55.3, 47.1, 31.3 (d, JF = 2.0 Hz), 23.1 (d, JF = 2.6 Hz), 22.4, 13.8. m/z [EI (+ve)] 337.2 [M]+, HRMS found [M]+ 337.1840, C22H24FNO requires 337.1842. IR (thin film) vmax = 2956, 2929, 2858, 1612, 1595, 1512, 1464, 1249 cm−1. m.p. 32–34 °C.
3-Fluoro-1-[(4′′-methoxyphenyl)methyl]-5-phenyl-2-(prop-2′-en-1′-yl)-1H-pyrrole, 13.
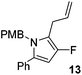
α,β-Unsaturated lactam 8 (41 mg, 0.14 mmol) was dissolved in diethyl ether (5 mL) and cooled to 0 °C. Allylmagnesium bromide (0.21 mL, 0.21 mmol, 1 M in Et2O) was added dropwise and the mixture was stirred for 1.5 h. Following this time, the reaction was quenched with H2O (10 mL), extracted with diethyl ether (3 × 10 mL), dried (Na2SO4) and evaporated in vacuo. The crude residue was purified be flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 13 (34 mg, 0.11 mmol, 75%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.30–7.12 (5H, m), 6.75 (4H, m), 5.98 (1H, s), 5.76 (1H, ddt, JH = 16.1, 10.1, 6.0 Hz), 5.00–4.84 (4H, m), 3.71 (3H, s, OCH3), 3.12 (2H, dd, JH = 5.9, 0.8 Hz). 19F NMR (CDCl3, 470 MHz) δ: −168.0. 13C NMR (CDCl3, 125 MHz) δ: 158.7, 149.5 (d, JF = 237.3 Hz), 135.3, 132.8, 130.8, 130.7, 128.9 (2C), 128.5 (2C), 127.2, 126.7 (2C), 115.4, 114.2 (2C), 113.9 (d, JF = 23.5 Hz), 96.6 (d, JF = 16.3 Hz), 55.3, 47.1, 27.5 (d, JF = 2.1 Hz). m/z [EI (+ve)] 321.1 [M]+, HRMS found [M]+ 321.1526, C21H20FNO requires 321.1529. IR (thin film) vmax = 2931, 1612, 1595, 1512, 1354, 1247, 1174 cm−1. m.p. 30–32 °C.
1-Benzyl-3-fluoro-5-phenyl-2,5-dihydro-1H-pyrrol-2-one, 16a.
Dialkene 15a (110 mg, 0.36 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–10% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 16a (100 mg, 0.35 mmol, 98%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.35–7.29 (3H, m), 7.26–7.20 (3H, m), 7.05–7.01 (4H, m), 6.19 (1H, d, JH = 1.9 Hz), 5.08 (1H, d, JH = 15.0 Hz), 4.70 (1H, dd, JF = 5.8 Hz, JH = 2.3 Hz), 3.53 (1H, d, JH = 15.0 Hz). 19F NMR (CDCl3, 470 MHz) δ: −138.6. 13C NMR (CDCl3, 125 MHz) δ: 163.0 (d, JF = 31.3 Hz), 152.4 (d, JF = 279.5 Hz), 136.5, 133.8, 129.3 (2C), 129.2, 128.8 (2C), 128.4 (2C), 127.8, 127.6 (2C), 118.5 (d, JF = 4.4 Hz), 59.3 (d, JF = 5.7 Hz), 44.1. m/z [ESI (+ve)] 290.1 [M + Na]+, HRMS found [M + Na]+ 290.0943, C17H14FNONa requires 290.0940. IR (thin film) vmax = 3063, 1710, 1666, 1456, 1220, 1186 cm−1.
3-Fluoro-1-[(4′-bromophenyl)methyl]-5-phenyl-2,5-dihydro-1H-pyrrol-2-one, 16b.
Dialkene 15b (120 mg, 0.31 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–5% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 16b (100 mg, 0.29 mmol, 96%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.36 (2H, d, JH = 8.3 Hz), 7.34–7.28 (3H, m), 7.03–7.00 (2H, m), 6.91 (2H, d, JH = 8.3 Hz), 6.21 (1H, d, JH = 2.0 Hz), 4.98 (1H, JH = 15.0 Hz), 4.69 (1H, dd, JF = 5.8 Hz, JH = 2.2 Hz), 3.54 (1H, JH = 15.0 Hz). 19F NMR (CDCl3, 470 MHz) δ: −138.5. 13C NMR (CDCl3, 125 MHz) δ: 163.0 (d, JF = 31.3 Hz), 152.3 (d, JF = 279.8 Hz), 135.5, 133.6 (d, JF = 2.2 Hz), 132.0 (2C), 130.1 (2C), 129.4 (2C), 129.3, 127.5 (2C), 121.9, 118.6 (d, JF = 4.4 Hz), 59.4 (d, JF = 5.6 Hz), 43.6. m/z [EI (+ve)] 344.9 [M]+, HRMS found [M]+ 345.0167, C17H13BrFNO requires 345.0165. IR (thin film) vmax = 2960, 1708, 1666, 1489, 1404, 1220, 1012 cm−1.
3-Fluoro-1-(cyclohexylmethyl)-5-phenyl-2,5-dihydro-1H-pyrrol-2-one, 16c.
Dialkene 15c (150 mg, 0.5 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–7.5% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 16c (130 mg, 0.46 mmol, 92%) as a pale yellow oil. 1H NMR (CDCl3, 500 MHz) δ: 7.37–7.22 (3H, m), 7.09–7.07 (2H, m), 6.20 (1H, d, JH = 1.5 Hz), 4.90 (1H, dd, JF = 5.5 Hz, JH = 2.0 Hz), 3.48 (1H, dd, JH = 14.0, 8.7 Hz), 2.46 (1H, dd, JH = 14.0, 6.0 Hz), 1.63–1.58 (2H, m), 1.40–1.43 (3H, m), 1.09–1.04 (3H, m), 0.87–0.79 (3H, m). 19F NMR (CDCl3, 470 MHz) δ: −138.4. 13C NMR (CDCl3, 125 MHz) δ: 163.3 (d, JF = 31.0 Hz), 152.6 (d, JF = 279.3 Hz), 134.2, 129.3 (2C), 129.1, 127.4 (2C), 117.9 (d, JF = 4.4 Hz), 60.7 (d, JF = 5.9 Hz), 46.6, 37.0, 30.9, 30.4, 26.3, 25.7, 25.6. m/z [EI (+ve)] 273.2 [M]+, HRMS found [M]+ 273.1528, C17H20FNO requires 273.1529. IR (thin film) vmax = 2922, 2852, 1703, 1666, 1448, 1220, 1116 cm−1.
3-Fluoro-1-[(1′-methyl-1H-pyrrol-2′-yl)methyl]-5-phenyl-2,5-dihydro-1H-pyrrol-2-one, 16d.
Dialkene 15d (80 mg, 0.27 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–5% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 16d (60 mg, 0.22 mmol, 81%) as a pale yellow solid. 1H NMR (CDCl3, 400 MHz) δ: 7.45–7.27 (3H, m), 7.09–7.06 (2H, m), 6.51 (1H, appt t, JH = 2.4 Hz), 6.18 (1H, d, JH = 2.0 Hz), 5.95 (1H, dd, JH = 3.6, 2.8 Hz), 5.78 (1H, dd, JH = 3.6, 2.0 Hz), 4.99 (1H, d, JH = 15.5 Hz), 4.74 (1H, dd, JF = 5.9 Hz, JH = 2.3 Hz), 3.60 (1H, d, JH = 15.5 Hz), 3.48 (3H, s). 19F NMR (CDCl3, 470 MHz) δ: −138.7. 13C NMR (CDCl3, 125 MHz) δ: 162.4 (d, JF = 31.3 Hz), 151.9 (d, JF = 279.8 Hz), 133.7, 129.3 (2C), 129.1, 127.6 (2C), 126.8, 123.3, 118.8 (d, JF = 4.2 Hz), 110.3, 106.9, 58.9 (d, JF = 5.5 Hz), 35.4, 34.1. m/z [EI (+ve)] 270.1 [M]+, HRMS found [M]+ 270.1170, C16H15FN2O requires 270.1168. IR (thin film) vmax = 2960, 2359, 1716, 1666, 1417, 1217 cm−1. m.p. 92–94 °C.
1-Benzyl-3-fluoro-2-methyl-5-phenyl-1H-pyrrole, 17a.
α,β-Unsaturated lactam 16a (33 mg, 0.13 mmol) was reacted with methyllithium (100 μL, 0.14 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 17a (29 mg, 0.11 mmol, 86%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.30–7.09 (8H, m), 6.87–6.84 (2H, m), 5.98 (1H, s), 4.99 (2H, s), 1.97 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −169.3. 13C NMR (CDCl3, 125 MHz) δ: 149.2 (d, JF = 235.6 Hz), 138.6, 132.8, 130.2 (d, JF = 3.3 Hz), 128.8 (2C), 128.8 (2C), 128.5 (2C), 127.2, 127.1, 125.6 (2C), 112.6 (d, JF = 24.4 Hz), 96.5 (d, JF = 16.5 Hz), 47.8, 8.2. m/z [EI (+ve)] 265.1 [M]+, HRMS found [M]+ 265.1269, C18H16FN requires 265.1267. IR (thin film) vmax = 2924, 1662, 1599, 1452, 1352, 1118 cm−1. m.p. 44–46 °C.
1-[(4′-Bromophenyl)methyl]-3-fluoro-2-methyl-5-phenyl-1H-pyrrole, 17b.
α,β-Unsaturated lactam 16b (38 mg, 0.11 mmol) was reacted with methyllithium (74 μL, 0.12 mmol, 1.6 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 17b (31 mg, 0.09 mmol, 83%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.36 (2H, d, JH = 8.6 Hz), 7.27–7.14 (5H, m), 6.72 (2H, d, JH = 8.6 Hz), 5.98 (1H, s), 4.93 (2H, s), 1.97 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −168.8. 13C NMR (CDCl3, 125 MHz) δ: 149.3 (d, JF = 236.2 Hz), 137.7, 132.6, 131.9 (2C), 130.2 (d, JF = 6.8 Hz), 128.8 (2C), 128.6 (2C), 127.3 (2C), 127.3, 121.0, 112.4 (d, JF = 24.4 Hz), 96.8 (d, JF = 16.5 Hz), 47.2, 8.1 (d, JF = 2.1 Hz). m/z [EI (+ve)] 343.1 [M]+, HRMS found [M]+ 343.0367, C18H15FNBr requires 343.0372. IR (thin film) vmax = 2924, 1680, 1599, 1489, 1363, 1072, 1010 cm−1. m.p. 98–100 °C.
1-(Cyclohexylmethyl)-3-fluoro-2-methyl-5-phenyl-1H-pyrrole, 17c.
α,β-Unsaturated lactam 16c (36 mg, 0.13 mmol) was reacted with methyllithium (91 μL, 0.15 mmol, 1.6 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 17c (29 mg, 0.11 mmol, 84%) as a white solid. 1H NMR (CDCl3, 500 MHz) δ: 7.31–7.28 (2H, m), 7.25–7.20 (3H, m), 5.81 (1H, s), 3.66 (2H, d, JH = 7.0 Hz), 2.17 (3H, d, JF = 1.6 Hz), 1.50–1.45 (3H, m), 1.28–1.23 (3H, m), 0.98–0.88 (3H, m), 0.56–0.49 (2H, m). 19F NMR (CDCl3, 470 MHz) δ: −170.1. 13C NMR (CDCl3, 125 MHz) δ: 149.1 (d, JF = 235.0 Hz), 133.9, 130.0 (d, JF = 7.2 Hz), 128.3 (2C), 128.3 (2C), 126.8, 112.2 (d, JF = 23.8 Hz), 96.3 (d, JF = 16.3 Hz), 50.2, 39.2, 30.4 (2C), 26.2, 25.7 (2C), 8.5 (d, JF = 1.9 Hz). m/z [EI (+ve)] 271.1 [M]+, HRMS found [M]+ 271.1733, C18H22FN requires 271.1736. IR (thin film) vmax = 2926, 2852, 1597, 1450, 1348, 1112 cm−1. m.p. 73–75 °C.
3-Fluoro-2-methyl-1-[(1′-methyl-1H-pyrrole-2′-yl)methyl]-5-phenyl-1H-pyrrole, 17d.
α,β-Unsaturated lactam 16d (31 mg, 0.12 mmol) was reacted with methyllithium (79 μL, 0.13 mmol, 1.6 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 17d (23 mg, 0.09 mmol, 75%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.28–7.23 (2H, m), 7.23–7.19 (3H, m), 6.46 (1H, appt t, JH = 2.5 Hz), 5.95 (1H, appt t, JH = 3.5 Hz), 5.92 (1H, s), 5.70–5.69 (1H, m), 4.88 (2H, s), 3.26 (3H, s), 2.03 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −169.5. 13C NMR (CDCl3, 125 MHz) δ: 149.2 (d, JF = 235.4 Hz), 133.0, 129.9 (d, JF = 6.9 Hz), 128.8 (3C), 128.5 (2C), 127.1, 122.3, 112.9 (d, JF = 24.6 Hz), 107.9, 107.1, 96.4 (d, JF = 16.5 Hz), 41.6, 33.7, 8.2. m/z [EI (+ve)] 268.1 [M]+, HRMS found [M]+ 268.1380, C17H17FN2 requires 268.1376. IR (thin film) vmax = 2922, 1705, 1599, 1469, 1361, 1301, 1089 cm−1. m.p. 69–71 °C.
1-Benzyl-3-fluoro-5-(4′-methoxyphenyl)-2,5-dihydro-1H-pyrrol-2-one, 21a.
Dialkene 20a (100 mg, 0.31 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–10% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 21a (70 mg, 0.22 mmol, 71%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.35–7.29 (3H, m), 7.14–7.13 (2H, m), 7.03 (2H, d, JH = 8.7 Hz), 6.93 (2H, d, JH = 8.7 Hz), 6.25 (1H, d, JH = 1.6 Hz), 5.15 (1H, d, JH = 15.0 Hz), 4.75 (1H, dd, JF = 5.8 Hz, JH = 2.1 Hz), 3.85 (3H, s), 3.61 (1H, d, JH = 15.0 Hz). 19F NMR (CDCl3, 470 MHz) δ: −138.7. 13C NMR (CDCl3, 125 MHz) δ: 162.9 (d, JF = 31.3 Hz), 160.3, 152.3 (d, JF = 279.3 Hz), 136.7, 128.9 (2C), 128.8 (2C), 128.4 (2C), 127.8, 125.4 (d, JF = 2.1 Hz), 118.5 (d, JF = 4.0 Hz), 114.6 (2C), 58.7 (d, JF = 5.8 Hz), 55.4, 43.9. m/z [EI (+ve)] 297.1 [M]+, HRMS found [M]+ 297.1169, C18H16FNO2 requires 297.1165. IR (thin film) vmax = 2933, 1707, 1666, 1512, 1247, 1174, 1030 cm−1. m.p. 101–103 °C.
1-Benzyl-3-fluoro-5-[(4′-trifluoromethyl)phenyl]-2,5-dihydro-1H-pyrrol-2-one, 21b.
Dialkene 20b (80 mg, 0.22 mmol) was treated with 15 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–15% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 21b (70 mg, 0.20 mmol, 88%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.58 (2H, d, JH = 8.1 Hz), 7.26–7.21 (3H, m), 7.16 (2H, d, JH = 8.1 Hz), 7.03–7.01 (2H, m), 6.19 (1H, d, JH = 1.2 Hz), 5.09 (1H, d, JH = 15.0 Hz), 4.77 (1H, dd, JF = 4.8 Hz, JH = 2.0 Hz), 3.57 (1H, d, JH = 15.0 Hz). 19F NMR (CDCl3, 470 MHz) δ: −62.9, −137.2. 13C NMR (CDCl3, 125 MHz) δ: 162.9 (d, JF = 31.2 Hz), 152.7 (d, JF = 280.9 Hz), 138.1, 136.1, 131.7, 131.4, 129.0 (2C), 128.4 (2C),128.1, 128.0 (2C), 126.3 (2C, q, JF = 3.7 Hz), 118.0 (d, JF = 5.0 Hz), 58.7 (d, JF = 5.7 Hz), 44.4. m/z [EI (+ve)] 335.0 [M]+, HRMS found [M]+ 335.0932, C18H13F4NO requires 335.0933. IR (thin film) vmax = 2362, 2332, 1718, 1670, 1421, 1325, 1166, 1126, 1066 cm−1.
1-Benzyl-3-fluoro-5-(4′-bromophenyl)-2,5-dihydro-1H-pyrrol-2-one, 21c.
Dialkene 20c (100 mg, 0.3 mmol) was treated with 10 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–10% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 21c (90 mg, 0.3 mmol, 94%) as a pale yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.54 (2H, d, JH = 8.4 Hz), 7.36–7.30 (3H, m), 7.13–7.10 (2H, m), 7.00 (2H, d, JH = 8.4 Hz), 6.26 (1H, d, JH = 1.6 Hz), 5.17 (1H, d, JH = 15.0 Hz), 4.76 (1H, dd, JF = 5.8 Hz, JH = 2.2 Hz), 3.62 (1H, d, JH = 15.0 Hz). 19F NMR (CDCl3, 470 MHz) δ: −137.7. 13C NMR (CDCl3, 125 MHz) δ: 162.9 (d, JF = 31.2 Hz), 152.5 (d, JF = 280.5 Hz), 136.3, 132.9 (d, JF = 2.2 Hz), 132.5 (2C), 129.2 (2C), 128.9 (2C), 128.4 (2C), 128.0, 123.2, 118.1 (d, JF = 4.7 Hz), 58.6 (d, JF = 5.7 Hz), 44.2. m/z [EI (+ve)] 345.1 [M]+, HRMS found [M]+ 345.0165, C17H13BrFNO requires 345.0165. IR (thin film) vmax = 3030, 1708, 1666, 1489, 1408, 1220, 1078, 1010 cm−1.
1-Benzyl-3-fluoro-5-cyclohexyl-2,5-dihydro-1H-pyrrol-2-one, 21d.
Dialkene 20d (100 mg, 0.34 mmol) was treated with 7.5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–5% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 21d (90 mg, 0.32 mmol, 95%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.28–7.16 (5H, m), 6.12 (1H, d, JH = 2.1 Hz), 5.03 (1H, d, JH = 15.2 Hz), 4.01 (1H, d, JH = 15.2 Hz), 3.72–3.70 (1H, m), 1.83–1.69 (2H, m), 1.66–1.54 (3H, m), 1.31–1.18 (2H, m), 1.05–0.97 (3H, m), 0.87–0.77 (1H, m). 19F NMR (CDCl3, 470 MHz) δ: −136.9. 13C NMR (CDCl3, 125 MHz) δ: 163.4 (d, JF = 31.5 Hz), 152.8 (d, JF = 277.4 Hz), 136.7, 128.3 (2C), 128.0 (2C), 127.7, 115.6 (d, JF = 4.2 Hz), 60.1 (d, JF = 4.4 Hz), 44.2, 37.8, 30.1, 36.5, 26.3, 25.7, 25.5. m/z [EI (+ve)] 273.2 [M]+, HRMS found [M]+ 273.1527, C17H20FNO requires 273.1529. IR (thin film) vmax = 2928, 2854, 1703, 1666, 1450, 1421, 1240, 1145 cm−1. m.p. 53–55 °C.
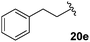
1-Benzyl-3-fluoro-5-(2′-phenylethyl)-2,5-dihydro-1H-pyrrol-2-one, 21e.
Dialkene 20e (120 mg, 0.38 mmol) was treated with 5 mol% Grubbs 2nd generation catalyst as described in General procedure I. The crude product was purified by flash column chromatography (0–10% EtOAc in petroleum ether) to yield the desired α,β-unsaturated lactam 21e (110 mg, 0.36 mmol, 96%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.26–7.11 (8H, m), 6.97–6.96 (2H, m), 6.17 (1H, d, JH = 1.6 Hz), 5.00 (1H, d, JH = 15.2 Hz), 4.06 (1H, d, JH = 15.2 Hz), 3.88–3.85 (1H, m), 2.49–2.31 (2H, m), 2.11–2.04 (1H, m), 1.86–1.75 (1H, m). 19F NMR (CDCl3, 470 MHz) δ: −137.0. 13C NMR (CDCl3, 125 MHz) δ: 163.1 (d, JF = 31.3 Hz), 152.7 (d, JF = 278.2 Hz), 140.4, 136.5, 128.9 (2C), 128.7 (2C), 128.2 (2C), 128.1 (2C), 127.9, 126.4, 117.2 (d, JF = 4.2 Hz), 55.0 (d, JF = 5.0 Hz), 44.3, 31.7 (d, JF = 2.0 Hz), 30.0. m/z [EI (+ve)] 295.2 [M]+, HRMS found [M]+ 295.1373, C19H18FNO requires 295.1372. IR (thin film) vmax = 2935, 2364, 1707, 1666, 1454, 1226 cm−1. m.p. 62–65 °C.
1-Benzyl-3-fluoro-5-(4′-methoxyphenyl)-2-methyl-1H-pyrrole, 22a.
α,β-Unsaturated lactam 21a (32 mg, 0.11 mmol) was reacted with methyllithium (83 μL, 1.2 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 22a (28 mg, 0.09 mmol, 88%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.25–7.16 (3H, m), 7.10 (2H, d, JH = 8.9 Hz), 6.86–6.84 (2H, m), 6.76 (2H, d, JH = 8.9 Hz), 5.91 (1H, s), 4.95 (2H, s), 3.71 (3H, s), 1.97 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −169.6. 13C NMR (CDCl3, 125 MHz) δ: 158.9, 149.1 (d, JF = 235.4 Hz), 138.7, 130.2 (2C), 129.9 (d, JF = 3.3 Hz), 128.8 (2C), 127.1, 125.6 (2C), 125.4 (d, JF = 1.6 Hz), 113.9 (2C), 111.8 (d, JF = 24.4 Hz), 96.0 (d, JF = 16.4 Hz), 55.3, 47.6, 8.1 (d, JF = 2.0 Hz). m/z [EI (+ve)] 295.2 [M]+, HRMS found [M]+ 295.1373, C19H18FNO requires 295.1372. IR (thin film) vmax = 2929, 1653, 1603, 1454, 1249, 1176 cm−1. m.p. 74–76 °C.
1-Benzyl-3-fluoro-2-methyl-5-[(4′-trifluoromethyl)phenyl]-1H-pyrrole, 22b.
α,β-Unsaturated lactam 21b (22 mg, 0.06 mmol) was reacted with methyllithium (51 μL, 0.07 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 22b (16 mg, 0.05 mmol, 73%) as a yellow solid. 1H NMR (CDCl3, 400 MHz) δ: 7.46 (2H, d, JH = 8.7 Hz), 7.32–7.17 (5H, m), 6.87–6.85 (2H, m), 6.05 (1H, s), 5.01 (2H, s), 2.00 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −62.5, −168.5. 13C NMR (CDCl3, 125 MHz) δ: 149.3 (d, JF = 236.4 Hz), 138.1, 136.3, 129.0, 128.9 (2C), 128.6 (d, JF = 7.2 Hz), 128.5 (2C), 127.4, 126.0 (d, JF = 3.8 Hz), 125.5, 125.4 (2C), 114.1 (d, JF = 24.4 Hz), 96.7 (d, JF = 16.5 Hz), 47.9, 8.2 (d, JF = 2.0 Hz). m/z [EI (+ve)] 333.2 [M]+, HRMS found [M]+ 333.1139, C19H15F4N requires 333.1141. IR (thin film) vmax = 2926, 1606, 1325, 1166, 1124 cm−1. m.p. 75–77 °C.
1-Benzyl-5-(4′-bromophenyl)-3-fluoro-2-methyl-1H-pyrrole, 22c.
α,β-Unsaturated lactam 21c (43 mg, 0.13 mmol) was reacted with methyllithium (98 μL, 0.14 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 22c (34 mg, 0.10 mmol, 78%) as a brown solid. 1H NMR (CDCl3, 400 MHz) δ: 7.37 (2H, d, JH = 8.6 Hz), 7.29–7.16 (3H, m), 7.04 (2H, d, JH = 8.6 Hz), 6.85–6.83 (2H, m), 5.97 (1H, s), 4.96 (2H, s), 1.98 (3H, d, JF = 1.6 Hz). 19F NMR (CDCl3, 470 MHz) δ: −168.9. 13C NMR (CDCl3, 125 MHz) δ: 149.2 (d, JF = 236.1 Hz), 138.3, 131.7 (d, JF = 1.8 Hz), 131.6 (2C), 130.2 (2C), 128.9 (2C), 128.8, 127.3, 125.5 (2C), 121.2, 113.2 (d, JF = 24.3 Hz), 96.9 (d, JF = 16.5 Hz), 47.7, 8.2 (d, JF = 2.0 Hz). m/z [EI (+ve)] 343.1 [M]+, HRMS found [M]+ 343.0368, C18H15BrFN requires 343.0372. IR (thin film) vmax = 2922, 1683, 1612, 1471, 1352 cm−1. m.p. 98–100 °C.
1-Benzyl-5-cyclohexyl-3-fluoro-2-methyl-1H-pyrrole, 22d.
α,β-Unsaturated lactam 21d (32 mg, 0.12 mmol) was reacted with methyllithium (92 μL, 0.13 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 22d (29 mg, 0.11 mmol, 92%) as a white solid. 1H NMR (CDCl3, 400 MHz) δ: 7.25–7.13 (3H, m), 6.80–6.76 (2H, m), 5.64 (1H, s), 4.90 (2H, s), 2.33–2.24 (1H, m), 1.91 (3H, d, JF = 1.6 Hz), 1.74–1.63 (4H, m), 1.26–1.08 (6H, m). 19F NMR (CDCl3, 470 MHz) δ: −170.6. 13C NMR (CDCl3, 125 MHz) δ: 148.6 (d, JF = 233.9 Hz), 138.7, 134.8 (d, JF = 5.7 Hz), 128.7 (2C), 127.1, 125.5 (2C), 109.3 (d, JF = 24.7 Hz), 91.8 (d, JF = 16.8 Hz), 46.4, 35.6, 34.1 (2C), 26.6 (2C), 26.0, 7.8 (d, JF = 2.1 Hz). m/z [EI (+ve)] 271.1 [M]+, HRMS found [M]+ 271.1737, C18H22FN requires 271.1736. IR (thin film) vmax = 2926, 2852, 1616, 1446, 1365, 1348, 1112 cm−1. m.p. 55–57 °C.
1-Benzyl-3-fluoro-2-methyl-5-(2′-phenylethyl)-1H-pyrrole, 22e.
α,β-Unsaturated lactam 21e (34 mg, 0.11 mmol) was reacted with methyllithium (91 μL, 0.12 mmol, 1.4 M in diethyl ether) following General procedure II. The product was purified by flash column chromatography (0–2.5% diethyl ether in petroleum ether) to yield the pyrrole 22e (24 mg, 0.08 mmol, 74%) as a yellow oil. 1H NMR (CDCl3, 400 MHz) δ: 7.24–7.08 (6H, m), 7.04–7.00 (2H, m), 6.80–6.76 (2H, m), 5.71 (1H, s), 4.83 (2H, s), 2.76–2.71 (2H, m), 2.64–2.60 (2H, m), 1.97 (3H, br s). 19F NMR (CDCl3, 470 MHz) δ: −170.5. 13C NMR (CDCl3, 125 MHz) δ: 148.3 (d, JF = 234.1 Hz), 141.4, 138.2, 128.8 (2C), 128.4 (2C), 128.3 (2C), 127.9 (d, JF = 6.3 Hz), 127.2, 126.1, 125.5 (2C), 110.0 (d, JF = 24.6 Hz), 94.1 (d, JF = 16.8 Hz), 46.5, 35.4, 28.4, 7.9 (d, JF = 2.1 Hz). m/z [EI (+ve)] 293.1 [M]+, HRMS found [M]+ 293.1581, C20H20FN requires 293.1580. IR (thin film) vmax = 2922, 1614, 1496, 1454, 1417, 1363, 1114 cm−1.
Acknowledgements
We would like to thank the EPSRC and AstraZeneca for postgraduate support (T. C.) and for a Leadership Fellowship (R. M.). The authors also thank Dr Ian Sword and the EPSRC (grant EP/H005692/1) for funding.
References
-
S. E. De Laszlo, N. J. Liverton, G. S. Ponticello, H. G. Selnick and N. B. Mantlo, U.S. Patent, US5837719A19981117, 1998 Search PubMed.
- H. Onda, H. Toi, Y. Aoyama and H. Ogoshi, Tetrahedron Lett., 1985, 26, 4221–4224 CrossRef CAS.
-
B.-Y. Zhu, T. Su, W. Li, E. A. Goldman, P. Zhang, Z. J. Jia and R. M. Scarborough, PCT Int. Appl, WO2002026734 A1 20020404, 2002 Search PubMed.
-
S. E. De Laszlo, N. J. Liverton, G. S. Ponticello, H. G. Selnick and N. B. Mantlo, U.S. Patent, US5837719A19981117, 1998 Search PubMed.
- W. W. Wikerson, W. Galbraith, K. Gans-Brangs, M. Grubb, W. E. Hewes, B. Jaffee, J. P. Kenney, J. Kerr and N. Wong, J. Med. Chem., 1994, 37, 988–998 CrossRef.
- P. R. Bovy, D. B. Reitz, J. T. Collins, T. S. Chamberlain, G. M. Olins, V. M. Corpus, E. G. McMahon, M. A. Palomo, J. P. Koepke, G. J. Smits, D. E. McGraw and J. F. Gaw, J. Med. Chem., 1993, 36, 101–110 CrossRef CAS PubMed.
- O. V. Serdyuk, V. M. Muzalevskiy and V. G. Nenajdenko, Synthesis, 2012, 2115–2137 CAS.
-
(a) F. C. Gozzo, D. R. Ifa and M. N. J. Eberlin, Org. Chem., 2000, 65, 3920–3925 CrossRef CAS;
(b) G. Shi and M. Schlosser, Tetrahedron, 1993, 49, 1445–1456 CrossRef CAS;
(c) Y. Wang and S. Zhu, Org. Lett., 2003, 5, 745–748 CrossRef CAS PubMed;
(d) H. Takaya, S. Kojima and S. Murahashi, Org. Lett., 2001, 3, 421–424 CrossRef CAS PubMed;
(e) J. Leroy, E. Porhiel and A. Bondon, Tetrahedron, 2002, 58, 6713–6732 CrossRef CAS;
(f) K. C. Kwak, H. Oh, D.-S. Chung, Y.-H. Lee, S.-H. Baik, J.-S. Kim and K. Y. Chai, J. Korean Chem. Soc., 2001, 45, 100–103 CAS;
(g) G. Shi and W. Cai, J. Org. Chem., 1995, 60, 6289–6295 CrossRef CAS;
(h) K. D. Barnes, Y. Hu and D. A. Hunt, Synth. Commun., 1994, 24, 1749–1755 CrossRef CAS;
(i) Z.-M. Qiu and D. J. Burton, Tetrahedron Lett., 1994, 35, 4319–4322 CrossRef CAS;
(j) Z.-M. Qiu and D. J. Burton, Tetrahedron Lett., 1995, 36, 5119–5122 CrossRef CAS;
(k) M. R. Grimmett, Adv. Heterocycl. Chem., 1993, 57, 291–295 CrossRef CAS;
(l) G. Buhr, Chem. Ber., 1973, 106, 3544–3558 CrossRef CAS;
(m) S. Kagabu, C. Ando and J. Ando, J. Chem. Soc., Perkin Trans. 1, 1994, 739–740 RSC.
-
(a) R. Surmont, G. Verniest, F. Colpaert, G. Macdonald, J. W. Thuring, F. Deroose and N. De Kimpe, J. Org. Chem., 2008, 74, 1377–1380 CrossRef PubMed;
(b) R. Surmont, G. Verniest and N. De Kimpe, Org. Lett., 2009, 11, 2920–2923 CrossRef CAS PubMed;
(c) B.-M. Kim, Q.-Z. San, L. R. Bhatt, D.-W. Jung, Y.-H. Lee and K.-Y. Chai, Bull. Korean Chem. Soc., 2009, 30, 1293–1296 CrossRef CAS.
-
(a) M. Marhold, A. Buer, H. Hiemstra, J. H. Van Maarseveen and G. Haufe, Tetrahedron Lett., 2004, 45, 57–60 CrossRef CAS;
(b) S. Fustero, A. Simon-Fuentes, P. Barrio and G. Haufe, Chem. Rev., 2015, 115, 871–930 CrossRef CAS PubMed.
-
(a) V. De Matteis, F. L. van Delft, R. Gelder, J. Tiebes and F. P. J. T. Rutjes, Tetrahedron Lett., 2004, 45, 959–963 CrossRef CAS;
(b) V. De Matteis, F. L. van Delft, J. Tiebes and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2006, 1166–1176 CrossRef CAS;
(c) V. De Matteis, O. Dufay, D. C. J. Waalboer, F. L. van Delft, J. Tiebes and F. P. J. T. Rutjes, Eur. J. Org. Chem., 2007, 2667–2675 CrossRef CAS;
(d) M. Marhold, C. Stillig, R. Fröhlich and G. Haufe, Eur. J. Org. Chem., 2014, 5777–5785 CrossRef CAS.
- T. J. Cogswell, C. S. Donald, D. Long and R. Marquez, Org. Biomol. Chem., 2015, 13, 717–728 CAS.
- M. T. Crimmins and M. Shamszad, Org. Lett., 2006, 9, 149–152 CrossRef PubMed.
- G. Verniest, S. Boterberg, F. Bombeke, C. V. Stevens and N. De Kimpe, Synlett, 2004, 1059–1063 CAS.
- P. S. Anderson, M. E. Christy, C. D. Colton, W. Halczenko, G. S. Ponticello and K. L. Shepard, J. Org. Chem., 1979, 44, 1519–1533 CrossRef CAS.
- G. Verniest, S. Claessens, F. Bombeke, T. Van Thienen and N. De Kimpe, Tetrahedron, 2005, 61, 2879–2887 CrossRef CAS.
- M. D'hooghe, C. Buyck, J. Contreras and N. De Kimpe, Org. Biomol. Chem., 2008, 6, 3667–3669 Search PubMed.
- E. Benedetti, M. Lomazzi, F. Tibiletti, J.-P. Goddard, L. Fensterbank, M. Malacria, G. Palmisano and A. Penoni, Synthesis, 2012, 3523–3533 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob02155c |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 full widths at half height. Flash chromatography was performed using silica gel (40–63 micron) as the stationary phase. TLC was performed on aluminium sheets pre-coated with silica (Silica Gel 60 F254) unless otherwise stated. The plates were visualised by the quenching of UV fluorescence (λmax 254 nm) and/or by staining with either anisaldehyde, potassium permanganate, iodine or cerium ammonium molybdate followed by heating.
000 full widths at half height. Flash chromatography was performed using silica gel (40–63 micron) as the stationary phase. TLC was performed on aluminium sheets pre-coated with silica (Silica Gel 60 F254) unless otherwise stated. The plates were visualised by the quenching of UV fluorescence (λmax 254 nm) and/or by staining with either anisaldehyde, potassium permanganate, iodine or cerium ammonium molybdate followed by heating.